The results of clinical studies by modern scientists indicate that various microelements directly or indirectly affect the hemostasis system of the human body. Iron is no exception, which also affects blood clotting. Microelement deficiency leads to an increased risk of thrombosis, that is, factors leading to aggregation are activated, or as it is also called, the gluing of formed elements. Scientists began studying changes in blood clotting factors. The results were somewhat surprising, since individuals with a hereditary predisposition to thrombotic pathologies also suffered from iron deficiency.
Preparing for analysis
The reliability of the blood test may well be affected by its preparation and implementation. Therefore, it is worth noting the main points of preparation in order to receive normal research results without false deviations.
- Eliminate heavy foods (fried, fatty and spicy foods) from your diet at least 24 hours before blood sampling - it is best to stick to a balanced diet several days before the test.
- Reduce the consumption of coffee, strong tea, psychostimulants to a minimum - 12 hours before donating blood, you should not take substances that affect the central nervous system (caffeine, alcohol).
- Provide comfortable conditions for your emotional state, avoid stress and physical activity.
- On the day of blood collection, you should not eat before the procedure.
According to the analysis, the doctor compares the results from the laboratory with generally accepted ones and determines the presence of a possible disease.
Advantages of the serological research method in the diagnosis of infections.
Serological diagnostics - fast and affordable
- High sensitivity and specificity.
- Wide range of detectable infections.
- Early diagnosis of infection.
- Possibility of monitoring the development of the disease.
- Speed and ease of execution.
- Possibility of using a minimum volume of test material.
- Research safety.
Biochemical blood test: normal indicators
For convenience, the norms of biochemical blood test indicators in adults are shown in the table:
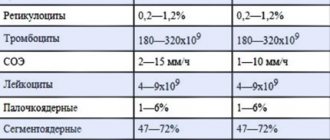
| Analysis: | Men: | Women: |
| Total protein | 64-84 g/l. | 64-84 g/l. |
| Hemoglobin | 130-160 g/l | 120-150 g/l. |
| Haptoglobin | 150-2000 mg/l | 150-2000 mg/l |
| Glucose | 3.30-5.50 mmol/l. | 3.30-5.50 mmol/l. |
| Urea | 2.5-8.3 mmol/l. | 2.5-8.3 mmol/l. |
| Creatinine | 62-115 µmol/l | 53-97 µmol/l. |
| Cholesterol | 3.5-6.5 mmol/l. | 3.5-6.5 mmol/l. |
| Bilirubin | 5-20 µmol/l. | 5-20 µmol/l. |
| AlAT (ALT) | up to 45 units/l. | up to 31 units/l. |
| ASAT (AST) | up to 45 units/l. | up to 31 units/l. |
| Lipase | 0-190 units/l. | 0-190 units/l. |
| Alpha amylase | 28-100 units/l. | 28-100 units/l. |
| Pancreatic amylase | 0-50 units/l. | 0-50 units/l. |
Each of the criteria indicated in the table reflects the condition of one or more human organs, and the combination of some of them allows in some cases to make an accurate diagnosis or direct the diagnostic process in the right direction.
Below we will look at what each of these tests shows using the example of deciphering a biochemical blood test in adults.
Total protein
Total protein is the total concentration of proteins found in the blood. Proteins take part in all biochemical reactions of the body - they transport various substances, act as catalysts for reactions, and participate in immune defense.
Normal levels of protein in the blood are 64-84 g/l. If the protein is higher than this, the body may be susceptible to infection. In addition, the cause of increased protein may be arthritis, rheumatism, or the onset of cancer. With a low protein content in the blood, the likelihood of liver disease increases many times, as well as problems with the intestines and kidneys. The most difficult diagnosis for low protein is cancer.
See in more detail: why protein is elevated in the blood.
Calcium
A trace element such as calcium is one of the most important minerals for us. It not only participates in the coagulation process, but also in other reactions to maintain normal hemostasis, the formation of a physiological state and the content of colloid and crystalloid elements. The influence of calcium on the digestibility of other metals is also very high.
Its other most important function is to regulate the permeability of cell wall membranes and blood vessels. It helps to compact them and reduce permeability. Sodium and potassium in this case have the opposite effect. Here it is necessary to draw an important conclusion that all microelements are closely related to each other, and an imbalance of one of them will entail a change in the concentration of the other, and the correction of this condition will not consist of one step.
Read also Thrombocytopenia of newborns
Calcium plays a role in the normal content of prothrombin, from which thrombin enzyme is formed. This process occurs only when there is a sufficient amount of calcium in the blood. Its excess can be considered one of the factors that increases coagulation.
It should also be noted that this trace element is extremely important for the normal excitability of tissues and nerve fibers. Excessive muscle activity leads to hypovolemia and micronutrient imbalance due to increased fluid loss and mineral consumption. The next stage of this pathogenetic mechanism will be hypercoagulation, which almost always occurs against the background of hypercalcemia.
Albumen
This protein is produced by the liver and is considered the main protein in the blood plasma. In general, experts distinguish albumins as a separate protein group, called protein fractions.
An increase in the concentration of albumin in the blood (hyperalbuminemia) may be associated with the following pathologies:
- dehydration, or dehydration (loss of fluid from the body through vomiting, diarrhea, profuse sweating);
- extensive burns.
A reduced albumin level is observed in smoking patients and in women during pregnancy and breastfeeding. In other people, a decrease in albumin may indicate various liver pathologies (for example, cirrhosis, hepatitis, or oncology), or intestinal inflammation of an infectious nature (sepsis). In addition, in case of heart failure or cancer, burns or fever, various injuries or drug overdose, the albumin in the blood will be lower than normal.
The significance of each buffer system
The bicarbonate buffer system (the most controlled among others) is especially important: with an excess of protons, a reaction occurs with bicarbonate ions (HCO3−) and H2CO3 - carbonic acid is formed. This is nothing more than a solution of carbon dioxide in water. Further, its amount decreases due to the breakdown of this acid with the formation of carbon dioxide, which is excreted by the lungs. The activity of this buffer is inextricably linked with pulmonary ventilation.
The hemoglobin buffer is dependent on lung function and is associated with oxygenation, that is, oxygen saturation. Oxygen potentiates this buffer, i.e. it is determined by the activity of the respiratory system.
The protein system is responsible for neutralizing metabolic products.
The concentration of phosphate buffer is concentrated mainly in the kidney tubules and intracellular space. The acid-base reaction of urine - dihydrogen phosphate (H2PO4) - depends only on it. But NaHCO3 is absorbed back into the kidney tubules.
Glucose (sugar)
The most common indicator of carbohydrate metabolism is blood sugar. Its short-term increase occurs during emotional arousal, stress reactions, pain attacks, and after eating. The norm is 3.5-5.5 mmol/l (glucose tolerance test, sugar load test).
- Sugar is elevated - diabetes, endocrine disorders, pancreatitis, pancreatic tumor, cerebral hemorrhage, chronic liver and kidney damage, myocardial infarction, cystic fibrosis.
- Sugar is low - damage to the liver and pancreas, hypothyroidism, stomach or adrenal cancer, arsenic poisoning or certain medications, alcohol intoxication.
Respiratory acidosis
Its essence is the accumulation of CO2 in the blood as a result of respiratory failure. This is hypercapnia and hypoventilation. It can develop as a result of a person being in conditions with a high CO2 content.
Hypoventilation is always associated with respiratory failure, resulting from depression of the respiratory center. The causes of the pathology are: infections, poisoning by sleeping pills, traumatic brain injuries, myasthenia gravis, chronic pulmonary pathologies.
The compensatory mechanisms that the body activates, trying to adjust the pH to neutral values, will never act in excess - this is controlled. And it means, for example, that in case of respiratory disorders, pH compensation will tend to normal, but will never exceed 7.4. It should be noted that full compensation is rarely achievable.
Uric acid
The main breakdown product of the main component of nucleic acids - purine bases. Since it is not used further in metabolic processes, it is excreted unchanged by the kidneys. The normal level in blood plasma is 0.16-0.44 mmol/l.
An increase in uric acid levels in the blood indicates:
- renal failure;
- leukemia, lymphoma;
- prolonged fasting;
- alcohol abuse;
- overdose of salicylates and diuretics.
A decrease in the level of uric acid in the blood can be observed during treatment with piperazine drugs, allopurinol, prebenecid, ACTH, sometimes with hepatitis, anemia.
See in more detail: why uric acid in the blood is elevated.
Types of violations
They develop in many pathological conditions, and regulatory mechanisms in such cases may not work. Depending on the pH shift, acidosis and alkalosis may develop. The reasons for the displacement are respiratory (respiratory) and metabolic (exchange) shifts. Accordingly, alkalosis or acidosis, respiratory or metabolic, develops. Systems for regulating the acid-base state of the blood tend to quickly eliminate the changes that have arisen, and in case of respiratory disorders, metabolic compensation mechanisms are activated to help, and in case of metabolic disorders, respiratory mechanisms are activated.
Urea
It is a consequence of the breakdown of proteins. The permissible amount of this substance in a person’s blood changes with age. Often, the level of urea goes through the roof in patients who have pathologies in their kidneys: doctors prescribe a similar blood test to diagnose and predict the disease.
A decrease in the level of urea in the blood can be triggered by reasons that are physiological (pregnancy, fasting, excessive exercise), or pathological (celiac disease, cirrhosis of the liver, heavy metal poisoning).
See in more detail: why urea in the blood is elevated.
Alanine aminotransferase (ALT, AlAt)
This indicator, along with AST, is used in medical practice for laboratory diagnosis of liver damage. Alanine aminotransferase is synthesized intracellularly, and normally only a small part of this enzyme enters the blood. When the liver is damaged (hepatitis, cirrhosis) as a result of cytolysis (cell destruction), this enzyme enters the blood, which is detected by laboratory methods.
The level of this transaminase may also increase during myocardial infarction and other conditions. An increase in ALT that exceeds an increase in AST is characteristic of liver damage; if the AST indicator increases more than the ALT increases, then this, as a rule, indicates problems with myocardial (heart muscle) cells.
See in more detail: why ALT is elevated.
Mechanism
Pathophysiology of the acid-base state - any tissue of a living working organism is always sensitive to pH shifts in any direction. If it is exceeded and the reaction is alkaline, cell destruction immediately begins, proteins fold (denature), enzymes are inactivated, and the body may die.
Blood electrolytes are acids, alkalis and salts, which, under the influence of water, break down into cations and anions. The constancy or regulation of the acid-base state occurs due to, as mentioned, buffer systems. Their main purpose is to counteract sharp fluctuations in proton content.
These solutions tend to keep the level of hydrogen ions constant even when acids or alkalis are added to them or when they are diluted. The composition of the buffer is a mixture of a weak acid with its base, but with a strong anion, that is, it is an acid-base pair. For example, such a system can be called carbonate acid: H2CO3 and NaHC03.
There are several main buffer systems that constantly operate and exist in the blood:
- Bicarbonate (a mixture of H2CO3 and HCO3) - occupies 53% of the buffer capacity of the blood and is the most powerful.
- Hemoglobin system - consists of oxygenated hemoglobin (weak acid) and non-oxygenated (or deoxyhemoglobin). This weak base is HHb-KHvO2) – 35%. Oxyhemoglobin releases 80 times more protons into the environment.
- The protein buffer system is, first of all, blood albumin, so it is the main one for the internal environment of cells. This buffer takes up only 5-7% of the blood capacity. It works thanks to the amphoteric properties of the protein. In an acidic environment, albumin becomes a cation, in an alkaline environment it acts as an acid. This property is called ionization ability.
- The phosphate system (diphosphate-monophosphate - NaH2PO4 and NaHPO4) - makes up 2-5% of blood plasma.
Aspartate aminotransferase (AST, AST)
A cellular enzyme involved in amino acid metabolism. AST is found in the tissues of the heart, liver, kidneys, nervous tissue, skeletal muscles and other organs. An AST blood test may show an increase in AST in the blood if the body has a disease such as:
- myocardial infarction;
- viral, toxic, alcoholic hepatitis;
- angina pectoris;
- acute pancreatitis;
- liver cancer;
- acute rheumatic carditis;
- heavy physical activity;
- heart failure.
AST is elevated in skeletal muscle injuries, burns, heat stroke, and as a result of cardiac surgery.
See more details: why AST is elevated.
Cholesterol
A component of fat metabolism, it is involved in the construction of cell membranes, the synthesis of sex hormones and vitamin D. There is total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol.
Degrees of increased cholesterol in the blood:
- 5.2-6.5 mmol/l – mild degree of increase in the substance, a risk zone for atherosclerosis;
- 6.5-8.0 mmol/l – a moderate increase, which is corrected by diet;
- over 8.0 mmol/l – a high level requiring drug intervention.
An increase in cholesterol levels in the serum or blood plasma is an argument in favor of atherosclerosis, hypothyroidism (low activity of the thyroid gland), chronic hepatitis, decompensated diabetes mellitus, obstructive jaundice.
This indicator decreases when:
- malignant liver tumors;
- cirrhosis of the liver;
- rheumatoid arthritis;
- hyperfunction of the thyroid and parathyroid glands;
- starvation;
- malabsorption of substances;
- chronic obstructive pulmonary diseases.
Bilirubin
Bilirubin is a yellow-red pigment that is formed when hemoglobin breaks down in the spleen, liver and bone marrow. Its normal level in the blood of children and adults is 3.4–20.5 µmol/l.
If the table with the examination results contains an elevated level of bilirubin, then the doctor can diagnose one of the following diseases in adults:
- cholelithiasis;
- pancreatic tumors;
- inflammatory diseases of the biliary tract.
If bilirubin is below normal, then the patient may have one of the following diseases:
- acute viral hepatitis;
- bacterial liver damage (leptospirosis, brucellosis, etc.);
- toxic hepatitis;
- medicinal product;
- neoplasms in the liver and primary biliary cirrhosis;
- hemolytic anemia of various etiologies.
Bilirubin, formed as a result of the breakdown of hemoglobin (indirect), is released into the blood, where it binds to albumin and is transported to the liver. In liver cells, bilirubin combines with glucuronic acid. This bilirubin bound to glucuronic acid is called direct bilirubin.
See in more detail: why bilirubin is increased in the blood.
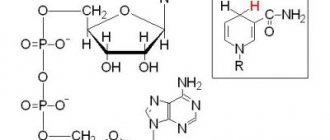
What ions affect blood clotting?
Our body's hemostasis system is designed to prevent blood loss or eliminate blockages in blood vessels. At its core, coagulation or blood clotting is a kind of internal protective mechanism. According to statistics, as of today, every second inhabitant of the planet suffers from a disruption of the hemostatic system.
They lead to such serious conditions as massive bleeding, ischemic disasters - heart attacks, strokes, thrombosis of the main arteries. If a person is not provided with emergency medical care, then the probability of death reaches 20-30%.
It is important to consider that most of these patients are simply unaware that they have coagulation disorders. Therefore, every doctor must examine his patients, and also know which trace element ions affect the functioning of this vital system of the body.
Amylase
Breaks down carbohydrates from food and ensures their digestion. Contained in the salivary glands and pancreas. There is alpha-amylysis (diastase) and pancreatic amylase.
- alpha-amylase rate: 28-100 units/l.
- pancreatic amylase rate: 0-50 units/l.
A high amylase content in a biochemical blood test indicates: peritonitis, pancreatitis, diabetes mellitus, pancreatic cyst, stone, cholecystitis or renal failure.
Decreased alpha-amylase: thyrotoxicosis; myocardial infarction; complete necrosis of the pancreas; toxicosis of pregnant women.
Potassium
Another important intracellular electrolyte. Its normal content in the body ranges from 3.5 to 5.5 mmol per liter.
Reduced potassium content:
- excess hormones of the adrenal cortex (including taking dosage forms of cortisone);
- chronic fasting (failure to receive potassium from food);
- prolonged vomiting, diarrhea (loss with intestinal juice);
- renal dysfunction;
- cystic fibrosis.
Increased potassium content:
- dehydration;
- acute renal failure (impaired renal excretion); ,
- adrenal insufficiency.
- cell damage (hemolysis - destruction of blood cells, severe starvation, convulsions, severe injuries).
The condition when potassium is elevated is called hyperkalemia, and when it is low, hypokalemia.
Sodium
Sodium does not directly participate in metabolism. It is completely abundant in the extracellular fluid. Its main function is to maintain osmotic pressure and pH. Sodium excretion occurs in the urine and is controlled by the adrenal hormone aldosterone.
Sodium reduction:
- decreased concentration due to increased fluid volume (diabetes mellitus, chronic heart disease)
- failure, liver cirrhosis, nephrotic syndrome, edema).
- loss of an element (abuse of diuretics, kidney pathology, adrenal insufficiency).
Increased sodium content:
- increased function of the adrenal cortex;
- excess salt intake;
- loss of extracellular fluid (profuse sweat, severe vomiting and diarrhea, increased urination in diabetes insipidus);
- violation of the central regulation of water-salt metabolism (pathology of the hypothalamus, coma).
An increase in a microelement is called hypernatremia, and a decrease is called hyponatremia.
A little physics
Any liquid can be characterized as acidic or alkaline. This depends and is determined by the content of the number of protons in it (the name of free hydrogen ions). The same applies to blood. Today, the acidity of any liquid medium is determined by such a concept as pH (power hydrogen). The scale and definition of pH (from 0 to 14) was introduced in 1908 by the Danish biochemist and physicist Søren Peter Lauritz Servisen.
The neutral reaction of a liquid - its pH - is equal to 7 units. Lower values indicate an increase in acidity; higher values turn the liquid alkaline.
The concept of the acid-base state of the body and its constancy is supported by 2 components - BR (buffer solutions or systems) and physiological compensation due to organs - kidneys, lungs, liver.