Counting red blood cells in the Goryaev chamber: formula with example
The Goryaev camera is an optical device necessary for calculating the number of cells in human biological fluids. The device is used in all medical institutions. He needs careful care so that the count of red blood cells in Goryaev’s chamber is reliable. The method is quite informative and convenient for laboratory doctors.
The device is represented by thick glass in which recesses are located. They are necessary for filling human biological fluid. A thin glass slide is placed on top, which helps eliminate air bubbles and distribute biological fluid throughout the recess.
The device allows you to count the number of white blood cells and red blood cells using a microscope aimed at deepening. The data obtained is substituted into a formula to determine the number of cells in a given volume of liquid.
Under a microscope, a laboratory doctor sees large and small squares, which add up to 225. In one study, it is necessary to count the cells in 100 squares.
Maintenance and care of the Goryaev camera
In order for the device to perform its functions, the laboratory doctor has correctly counted the formed elements; it must be looked after. It must always be clean and sterile. The presence of foreign objects will complicate the examination.
During the work when counting red blood cells in the Goryaev chamber, the following disinfecting manipulations are performed:
- using a gauze cloth dipped in an alcohol solution, wipe all the recesses of the chamber;
- Use a dry gauze cloth to wipe the surface again;
- the device is ready for use if rainbow circles appear on both sides.
After finishing work, the device is disinfected again:
- biological fluid is removed;
- the chamber is placed in an alcohol solution for half an hour; if other disinfectants are used, the time is extended to 60 minutes;
- take out the device, wipe it dry until rainbow circles appear;
- The glass is left on a dry surface.
Algorithms for counting red blood cells in the Goryaev chamber
Capillary blood is obtained by puncturing the fingertip with a scarifier. It is important that the first drop does not fall into the capillary, since it contains tissue fluid and damaged red blood cells. Collect blood into a capillary.
Next, manipulation is carried out to conduct research using the Goryaev camera.
- The blood must be diluted 200 times. To do this, use a sodium chloride solution.
- The chamber is prepared for analysis by wiping it with an alcohol solution and a dry gauze cloth. You cannot use cotton wool for these purposes, as its fibers may remain on the glass, which will complicate the analysis.
- A cover glass is placed on top of the chamber. They do this carefully so that it fits exactly into the grooves.
- Using a glass rod, take a small sample of biological fluid and bring it to the holes of the recesses. The blood should slowly spread throughout the chamber. Wait 1-2 minutes for the bubbles to disappear.
- The microscope is set to low magnification and the number of red blood cells in 80 small grid squares is counted.
- The resulting number is substituted into the formula, calculating the number of red blood cells per given volume of biological fluid.
The method is quite informative, but errors are possible. The doctor may make a mistake in the calculations using a microscope, or take a volume of liquid that does not contain enough red blood cells.
Therefore, if errors or inaccurate results appear, the therapist can prescribe repeated tests on a semi-automatic analyzer, which calculates the exact number of formed elements in 1 ml of liquid.
The advantage of the method is that the doctor can detect other formations in addition to red blood cells, which will help identify disorders in the body.
Formula for counting the number of red blood cells in Goryaev's chamber
After obtaining the number of red blood cells, the number must be substituted into the formula. It is used to calculate the number of red blood cells per given volume of biological fluid.
X=(E×4000×200)/80
- X is the number of red blood cells in a given volume of biological fluid;
- E – the amount of red blood cells detected under a microscope;
- 200 – blood dilution;
- 80 is the number of small squares in which the cells were counted.
To calculate red blood cells per 1 liter of blood, the result is multiplied by 1012.
Red blood cell norm and deviations
Normally, the number of red blood cells differs by gender and age colon
- men 3.8-5×1012;
- women 4-4.5×1012;
- children 5.5-7×1012.
The number of red blood cells may be increased or decreased. There are physiological reasons for the change. For example, in a woman during menstruation, the number of red blood cells is reduced because a certain amount of biological fluid is lost.
Pathological causes of increased red blood cells:
- dehydration, as a result of which the amount of blood plasma decreases, the number of formed elements increases;
- cancer of the blood, kidneys, endocrine glands, changing the hormonal balance in the body;
- lack of oxygen in the blood, resulting in red bone marrow producing red blood cells excessively.
Pathological causes of decreased red blood cells:
- large blood loss;
- periodic blood loss due to minor bleeding from the mucous membranes of the gastrointestinal tract;
- nutritional factor (consumption of food with a small amount of nutrients, trace elements, minerals);
- impaired absorption of substances in the intestine;
- increased blood plasma content;
- destruction of red blood cells as a result of blood transfusion, poisoning;
- chronic alcoholism, which impairs the functioning of the kidneys and red bone marrow, resulting in the formation of a small number of reticulocytes (precursors of red blood cells).
Using a Goryaev camera, the number of red blood cells is counted. It is important to know that there are more informative research methods that eliminate the risk of medical error.
The device works accurately if the methods for its disinfection are followed, the doctor carefully counts all the cells.
After receiving the test results, you must consult a doctor; it is impossible to make a diagnosis yourself.
Read a detailed review of all methods for counting the number of red blood cells from a doctor.
Ekaterina Belikova, laboratory diagnostics doctor, especially for Mirmam.pro
Source: https://mirmam.pro/podschet-eritrotsitov-v-kamere-goryaeva
Taking blood and counting red blood cells and white blood cells in a counting chamber
To conduct these studies, the following workplace equipment is required:
- Mixers for red blood cells and white blood cells.
- Test tubes of the serological type.
- Pipettes for 1 and 5 ml.
- Capillaries for Sali's hemometer.
- Glass rods.
- Eye droppers.
- Goryaev's counting chambers with ground glass for them.
- Microscopes.
- Cones with clean water.
- Salt.
- Corrosive sublimate.
- Sodium sulfate.
- Glacial acetic acid.
- 96° ethyl alcohol.
- Gentian violet (methylene blue).
- Finger blood collection kit.
Taking blood into mixers for counting red blood cells and white blood cells
To count the number of red blood cells and white blood cells, the following reagents are required:
- 3% sodium chloride solution (NaCl): 3 g of sodium chloride is placed in a 100 ml flask and topped up with distilled water to the 100 ml mark.
- Gayem's reagent: add 200 ml of distilled water to 0.5 g of sublimate, 1 g of sodium chloride and 5 g of sodium sulfate. Both reagents preserve red blood cells.
- 3% acetic acid solution - 3 ml of glacial acetic acid is placed in a 100 ml cylinder and topped up with distilled water to the mark. The reagent is tinted with a 1% aqueous solution of gentian violet at the rate of 5-6 drops of paint per 10 ml of 3% acetic acid.
Blood for counting red blood cells and white blood cells is taken into special mixers or test tubes.
A rubber tube with a glass mouthpiece is put on a clean and dry mixer for red blood cells, after which blood is sucked into it, without air bubbles, exactly to the 0.5 mark, then with the same mixer a 3% sodium chloride solution or Gayem's reagent is drawn exactly to the 101 mark, avoiding the appearance of air bubbles.
In this case, the blood is diluted 200 times. Remove the rubber tube from the mixer, grab it between your fingers, shake it several times, pierce a piece of paper with the patient’s name on it, and place it on the table in a horizontal position.
Blood is sucked into a clean dry mixer for leukocytes in the same way as into a mixer for erythrocytes to the 0.5 mark, after which a 3% acetic acid solution is drawn with the same mixer to the 11 mark.
In this case, the blood is diluted 20 times.
Then remove the rubber tube from the mixer, shake it several times, pierce it with a piece of paper on which the patient’s name is indicated, and place it on the table in a horizontal position.
Taking blood into test tubes for counting erythrocytes and leukocytes according to N. M. Nikolaev
For each blood draw, two numbered tubes are taken, serological and agglutination. 4 ml of a 3% solution of sodium chloride or Gayem’s reagent are placed in a serological tube, and 0.4 ml of a 3% solution of acetic acid is placed in an agglutination tube. Add 0.02 ml of blood taken with a capillary from a hemometer into each test tube, and immediately mix the contents of the tubes by rotating them between the palms.
In a serological test tube (for red blood cells), blood is diluted 200 times, and in an agglutination test tube (for leukocytes) - 20 times.
Studying Goryaev's counting chamber and filling it with blood to count red blood cells and white blood cells
The number of red blood cells and white blood cells is counted in special counting chambers. For this purpose, they use Goryaev’s counting chamber, which is glass with two grids applied to it.
The grids are separated from one another by recesses and are divided by dividing lines into 225 squares. The mesh area is 9 mm2, the height created by grinding the ground cover glass in the chamber is 0.1 mm, the volume is 0.9 mm3.
Before filling the chamber with blood, it is washed with water and wiped dry.
A ground cover glass is rubbed onto the dry surface of the chamber, smoothly moving it back and forth along it until colored Newton's rings appear at the points of contact of the cover glass with the chamber glass.
Then the blood in the mixer is shaken for 1-2 minutes, after which 1-2 drops of blood are released from the mixer onto the cotton wool, the next drop is placed in front of a polished cover glass.
When applying a drop of blood, care must be taken to ensure that no air gets into the space above the chamber mesh and that there is no excess liquid. One grid is filled to count red blood cells, the second - to count white blood cells.
To fill Goryaev’s chamber with blood taken according to N. M. Nikolaev’s method (test tube method), proceed as follows. Ground glass is ground to the counting chamber.
From each test tube, after shaking it first, using a glass rod with a melted end, take one drop of liquid and fill both chamber meshes separately with them, for which the drop is placed in front of the gap formed between the ground glass and the counting chamber, as a result of which the liquid fills the space above grids.
Counting erythrocytes and leukocytes in the Goryaev counting chamber
The number of erythrocytes and leukocytes in the Goryaev chamber is counted using a microscope. To do this, Goryaev’s chamber filled with diluted blood is placed on the microscope stage and the chamber grid is found under low magnification (15X or 10X eyepiece, 8X objective), with the condenser lowered. The counting of formed elements begins 2-3 minutes after the chamber is filled with blood.
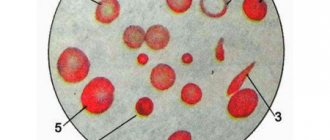
Red blood cells are counted in five squares, each of which is divided into 16 small squares. Counting begins from the upper left square, and then the camera moves diagonally from top to bottom.
To avoid repeated counting of the same red blood cells, they are guided by the following rule: all red blood cells inside the square and on the dividing lines are counted only when more than half of them go inside the square; cells crossed by a dividing line in half are counted only on two sides of the four sides of the square (on the left and top); cells that extend most of their half beyond the boundaries of the boundary lines are not counted at all. The results of counting red blood cells for each of the five large squares are recorded and summed up. To calculate the number of red blood cells, use the following formula:
where x is the number of red blood cells in 1 mm3 of blood; a is the sum of red blood cells counted in five squares; b — dilution of blood 200 times; c - the number of small squares counted - 80.
To simplify the calculation, four zeros are added to the number of counted red blood cells. Normally, the number of red blood cells is from 4,500,000 to 5,000,000 in 1 mm3 of blood. Leukocytes are counted in 100 large squares.
When counting them, they are guided by the same rules as those indicated for red blood cells, and then the calculation is made using the above formula, in which the dilution number is 20 and the number of squares counted is 1600.
For ease of calculation, the number of white blood cells counted is multiplied by 50 or divided by 2 and assigned two bullets.
Normally, the number of leukocytes is from 5000 to 8000 in 1 mm3 of blood.
Source: https://ginekolog.my1.ru/publ/klinicheskie_issledovanija/blood/podschet_ehritrocitov_i_lejkocitov_v_schetnoj_kamere/39-1-0-756
Counting leukocytes and erythrocytes in the Goryaev chamber (methodology)
Goryaev's camera
The Goryaev camera is a simple device for visually counting blood cells under microscopic magnification. It is constructed on the basis of a glass slide with special recesses for the nominal blood volume, as well as a cover glass with an applied grid.
There are several designs of the Goryaev chamber depending on the number of chambers (recesses for filling with blood); most often in laboratories two-chamber or four-chamber models are used. Above each of the chambers there is a special counting grid applied to the cover glass using laser engraving or micro-spray paint.
The device is designed in such a way that all available chambers have an equal volume, which is indicated in the technical documentation of the model; their depth and the dimensions of the sectors of the cover mesh are also standard. This makes it possible to convert, after counting the formed elements, their total values into units of concentration of given cells in the blood volume.
Description of the Goryaev camera grid
Grid in Goryaev's chamber
The cover glass is engraved in the form of a grid, the large squares of which have a side size of 1 mm. Most often, there are 9 such squares above each counting chamber; they are arranged so that they form three columns of three squares each.
Those large squares that are located along the periphery are divided into 16 smaller squares, the length and width of which are 0.2 mm.
The standard depth of the chamber under these squares makes it easy to count the number of cells in volume units; for them it is equal to 0.1 mm3 or 10-4 ml.
The large square located in the middle has additional dividing lines, they are drawn in such a way that they divide each of the 16 middle squares into 16 smallest ones. Their side is 0.05 mm.
Egorov's rule for counting shaped elements
Correct counting of cells in the grid
The counting of blood cells is carried out under a microscope using appropriate magnification, most often a x10 eyepiece is used. Count the number of cells in those large squares that are divided into 16 middle ones, start counting from the top left and move down to the right, capturing 5 large squares when counting.
In order to correctly determine the number of cells in a given volume of blood, it is necessary to exclude repeated counting of those formed elements that are located at the boundaries or nodes of the grid.
Such an error would lead to an increase in the calculated indicator; to eliminate it, Egorov’s rule was formulated.
It states that a given square includes those elements that lie within its boundaries, without touching the drawn lines, as well as those cells that are located on the top and left sides of the square. Those cells that “touch” the bottom or right side should not be taken into account when counting.
Method of counting leukocytes
In case of an analyzer error, Goryaev’s camera helps
To count leukocytes in the blood in modern clinical laboratories, special automatic analyzers are most often used; the counting technique using the Goryaev camera is rarely used due to its labor intensity and low speed of analysis. In order to correctly determine the concentration of individual types of leukocytes, a laboratory diagnostics doctor or medical assistant laboratory technician with skills in working with a microscope is required.
Counting using the Goryaev camera is justified in cases where the automatic analyzer is not able to correctly identify the patient’s blood cells.
This happens with cancer of the blood system and some other diseases in which the cells have an atypical appearance and volume.
The advantages of visual counting include the possibility of in-depth diagnostics due to the fact that an experienced doctor is able to distinguish altered cells characteristic of individual pathologies and reflect their presence in the analysis results.
Leukocytes must be prepared before work
To count certain types of leukocytes, blood is diluted several times with a weak solution of acetic acid, while samples without leukocytosis, as a rule, should be diluted at least 20 times; in cases of a pronounced increase in the number of white blood cells, greater dilution may be required. Methylene blue staining is used to contrast the image.
After filling the chambers with the test volume of blood, a cover glass with a mesh is placed on top and it is ground in, during which air bubbles between the two glasses should completely disappear. The filled chamber is placed under a microscope, and it is advisable to leave it motionless for 1-2 minutes so that the formed elements completely stop moving and settle.
After this, at medium magnification (eyepiece no less than x10), individual types of leukocytes are counted in at least 100 squares.
To convert the resulting number into volumetric values, you need to multiply it by the dilution (usually 20 times) and by the number of squares involved in the count (usually 100).
The resulting number gives an idea of the number of leukocytes in 1 μl of blood.
Method of counting red blood cells
Compliance with the algorithm allows for accurate cell counting
The blood being tested is diluted with saline solution at least 100 times so that a visual count of red blood cells becomes possible. If the dilution is insufficient, a double layer of blood cells forms in the chambers, making their determination impossible.
After applying a cover glass and grinding, microscopy of the preparation is carried out at a magnification of x10 eyepiece. Counting is carried out in all 5 large squares, divided by lines into 16 sectors.
Volumetric values are calculated by multiplying the resulting number of red blood cells by the degree of dilution (usually 100 or 200 times) and by 4000, then dividing the resulting number by 80 (the number of small squares when counting). The resulting number characterizes the number of red blood cells in 1 μl of blood.
Source: https://gidanaliz.ru/poznavatelno/podschet-v-kamere-gorjaeva.html
Egorov's rule for counting shaped elements
Correct counting of cells in the grid
The counting of blood cells is carried out under a microscope using appropriate magnification, most often a x10 eyepiece is used. Count the number of cells in those large squares that are divided into 16 middle ones, start counting from the top left and move down to the right, capturing 5 large squares when counting.
In order to correctly determine the number of cells in a given volume of blood, it is necessary to exclude repeated counting of those formed elements that are located at the boundaries or nodes of the grid. Such an error would lead to an increase in the calculated indicator; to eliminate it, Egorov’s rule was formulated. It states that a given square includes those elements that lie within its boundaries, without touching the drawn lines, as well as those cells that are located on the top and left sides of the square. Those cells that “touch” the bottom or right side should not be taken into account when counting.
Counting red blood cells in the Goryaev chamber - how is the level of red blood cells calculated?
The composition of blood is formed by plasma and formed components, most of which are red blood cells. Mature blood cells do not contain a nucleus and have a biconcave shape.
their function is to transport gases in organ tissues.
The level of red blood cells in a person’s blood depends on factors such as gender and age, but is a fairly constant value, so fluctuations in their number provide valuable information in the diagnostic process.
To calculate the number of blood cells, a unified method of counting red blood cells is most often used using equipment known as a Goryaev chamber, or using automatic electronic counters. Both methods are currently widely used both in daily laboratory practice and in scientific research, since the error in the results obtained during their use is minimal.
The structure of Goryaev's chamber
An optical device - the Goryaev camera - allows you to calculate the number of blood cells in a certain volume of a special liquid. The idea of creating the device belongs to the Russian professor N.K. Goryaev.
The main element of the device is a transparent glass slide with a chamber formed by a rectangular-shaped recess, the surface of which is covered with a special microscopic mesh, and a thin cover glass. High accuracy of counting results is achieved due to the increased mesh volume compared to similar devices of other types.
The mesh of the device has an area of 9 mm2, the size of one side is 3 mm. The surface of this element is formed by squares with a total number of 225 pieces. Of these, one hundred squares are divided into rectangles, the same number are not graphed, and 25 pieces are divided into 16 smaller ones. The area of 1 small square is 1/400 mm2, the area above it has a volume of 1/4000 mm3.
Diluting blood for research purposes
Determining the number of red blood cells consists of several sequential steps. The first step is diluting a certain volume of blood in the reagent. This is done due to the huge number of shaped elements.
The reagent most often used is a solution of sodium chloride with a concentration of 0.9%. If necessary, an alternative is 5% sodium citrate solution. The blood dilution procedure is performed in a test tube or in a special mixer called a melanger.
Attention! The use of solutions with higher or lower concentrations is excluded, as this will lead to the destruction of blood cells.
Dilution of blood in melangeur
Melanger is a 10 cm long graduated capillary made of glass with an extension at the end, inside of which there is a ball. The latter performs an important function - uniform mixing of the solution and blood. The color of the melange capillary ball will be red if it is intended for red blood cells, and white if it is intended for working with leukocytes.
The red blood cell counting technique allows the research procedure to be carried out with both stabilized blood and unstabilized material immediately after it is collected from the patient.
Blood is drawn with a melange capillary to a division of 0.5, after which the tip of the mixer is cleared of blood using gauze or cotton wool and the next step is to draw the dilution solution to a division of 101.
Covering the capillary holes with your fingers, the resulting sample is thoroughly mixed for about 3 minutes. The fourth drop is placed in the counting chamber, after first removing the first three onto gauze.
This results in a 200-fold dilution of the blood.
Diluting blood in a test tube
With the test tube method of blood dilution, the test material is diluted 200 times as follows: 4 ml of sodium chloride solution is placed in a test tube, after which 0.02 ml of blood is added.
To do this, the material is taken with a pipette, the tip of which is wiped with a cotton swab or other cloth. The blood sample is placed at the bottom of the tube, the pipette is washed close to the surface with a sodium chloride solution. The finished sample is mixed and left until the next stage of the study, but for less than 3 hours.
Red blood cell count
Goryaev's camera must be positioned horizontally. The entire grid area is carefully filled with the diluted sample. It is important to ensure that no air bubbles form, as their presence will significantly distort the result. The chamber remains in this position for one minute to allow the blood cells to settle.
The unified method of counting red blood cells is performed at medium magnification through microscopic examination. The number of red blood cells in Goryaev's chamber is counted in 5 large squares, divided into 16 small ones.
It is recommended to start counting from the large square located at the top left, and then diagonally.
Important! To prevent the number of cells located on the lines of small squares from being counted twice, it is recommended to use Egorov’s rule: in each square, count along two specific lines (top and left).
Start counting cells in small squares in the top row from left to right from 1st to 4th in this row. Moving to the next row, counting is carried out in the reverse order - from right to left. The transition to counting cells in the next large square is made after calculating the red blood cells in these 16 small squares.
The number of red blood cells is directly calculated using the formula shown in Figure 2.
The designations in this formula are deciphered as follows:
- X is the required number of red blood cells in 1 μl of blood,
- A denotes the number of cells counted,
- 200 – blood dilution,
- 80 is the number of small squares counted,
- 1/4000 is the volume of a small square.
Attention! To ensure the reliability of the result, the calculation of blood cells for some types of anemia must be carried out immediately after collecting the material.
Possible reasons for distorted results
Like any other laboratory test, counting red blood cells requires careful preparation and accuracy in execution. Distortion of analysis results can occur due to a number of reasons. The equipment is stored in a dry place. Any laboratory work related to blood is performed with rubber gloves.
First, you need to make sure that the camera is properly prepared and used. Before and after work, the device must be thoroughly rinsed with distilled water and wiped dry with a soft cloth. The coverslip must be prepared in advance in the same way.
Possible reasons affecting the reliability of the result:
- Poor quality reagents that can cause hemolysis.
- Formation of a blood clot in the material.
- Inaccuracy in the process of drawing blood into the capillary.
- Poorly processed tubes and pipettes.
- Unscrupulous technique when working with the camera and counting red blood cells.
- Counting cells immediately after filling the chamber (you need to wait 1 minute so that they have time to settle on the surface).
Recently, the method of counting red blood cells using automatic electronic counters has become increasingly popular. This trend is quite justified, since the automated research process is much more accurate and less labor-intensive.
The operating principle of this equipment is based on the different electrical conductivity of blood cells and the solution in which they are placed. A special electromagnetic device detects changes in the resistance level of the electrical circuit when red blood cells move through the opening of the capillary. Within a few seconds, the result with the calculated number of cells is displayed on the digital display.
An elevated red blood cell count in the test is called polycythemia. This condition occurs due to pathologies characterized by loss of fluid in the body, for example, diarrhea syndrome.
Often this phenomenon is observed in the first stages of infectious diseases, poisoning with toxic substances, etc. A decrease in the concentration of red blood cells in the blood is known as oligocythemia.
And often this pathology manifests itself during various types of anemia.
Loading…
Source: https://dlja-pohudenija.ru/serdcze/metod-podscheta-kolichestva-eritroczitov-v-krovi-s-pomoshhyu-kamery-goryaeva
Red blood cell count and clinical evaluation of results
Erythrocytes, as well as leukocytes and platelets, belong to the formed elements of blood. Their number is calculated during a general clinical blood test along with the assessment of ESR, determination of hemoglobin concentration, determination of a leukogram and calculation of red blood indices, which include the color index (CI) and hemoglobin content in one red blood cell (HGE).
Red blood cells are the most numerous formed elements of blood that contain hemoglobin. Consequently, their main function in the body is to carry out gas exchange. With the help of hemoglobin, red blood cells transport oxygen and carbon dioxide. In addition, red blood cells deliver amino acids and lipids to cells, take part in the regulation of acid-base balance, perform protective and other vital functions, which were discussed in more detail in the physiology course.
The content of erythrocytes in the blood of healthy animals and poultry is quite constant, so establishing changes in their number has diagnostic significance. True, fluctuations in their number can be observed depending on the time of day of the study, age, gender, productivity, and physical activity of the animal. For example, the number of red blood cells during the day is slightly lower than in the evening; in newborns their content is higher than in adults, as well as in males compared to females. Highly productive cows have higher erythrocyte content than low-producing cows. It has also been established that in horses after physical activity (for example, a 10-minute trot - Domrachev's test), the number of red blood cells increases by 20 percent or more.
All these factors must be taken into account when clinically assessing the results of red blood cell count determination. Several methods have been proposed for counting red blood cells, of which two are unified in medical practice:
1. Counting in a counting chamber using a microscope;
2. Counting using electronic automatic meters. Previously, methods were used to determine the number of red blood cells using an erythrosediometer (a graduated tube for determining ESR according to Nevodov), an erythrohemometer and a photoelectrocolorimeter, however, due to their low accuracy, they are currently not used. The error of the method of counting formed elements in the chamber is + 2-5%, and in automatic analyzers it is even less - up to + 2%. This predetermines the widespread use of these methods not only in laboratory practice, but also in scientific research.
Counting red blood cells in the chamber.
The principle of the method is that a precise amount of blood is mixed evenly with a specific amount of liquid. The diluted blood is placed in a chamber with a known volume. A mesh is applied to the bottom of the chamber, thanks to which accurate counting of red blood cells is possible through microscopy.
Several counting chambers have been proposed, of which the most widely used in the countries of the former USSR is the counting chamber with the Goryaev stack. It is often called “Goryaev’s camera,” although in fact it is Bürker’s camera with Goryaev’s grid (1910).
The counting chamber is a thick glass slide with four transverse grooves, between which there are three planes. The middle plane is 0.1 mm thinner than the side ones and is divided by a longitudinal groove into two equal halves, on each of which the Gryaev mesh is engraved. If you place a cover glass on the side planes of the camera and rub it until rainbow rings appear, the so-called. “Newton’s rings”, then above the middle plane there will be a slot-like space 1/10 mm high.
The Goryaev mesh has a size of 3x3 mm, i.e. its area is 9 mm2. It contains 225 large squares, 25 of which are divided into 16 small ones, 100 squares are not marked and another 100 are divided into rectangles. The area of one small square is 1/400 mm2, and the volume of the chamber above it is 1/4000 mm3.
As for the technique of counting red blood cells, the following sequence of steps is followed: 1) blood dilution; 2) filling the counting chamber; 3) cell counting itself; 4) calculation of the absolute number of red blood cells.
Stage 1
. Since the blood contains a significant amount of red blood cells, for example, a cow weighing 400 kg has more than 125 billion cells, it is possible to determine their number only after diluting the blood. For dilution, a 0.85-3% sodium chloride solution or a 5% sodium citrate solution is often used. The use of solutions of higher or lower concentrations is unacceptable, as it will lead to the destruction of red blood cells.
Blood is diluted either in a melanger-mixer for red blood cells, or in a test tube (according to N.M. Nikolaev, 1954). Melanger is a capillary tube 10 cm long with a spherical expansion. Inside the expansion there is a ball that promotes uniform mixing of blood and fluid. Since melangeurs are proposed for both erythrocytes and leukocytes, the color of this ball can be different: a red ball is a melanger for erythrocytes, a white one is for leukocytes.
For the study, both stabilized blood and unstabilized blood are used immediately after collection. Blood is drawn into the melanger to the “0.5” or “1” mark. The end of the mixer is cleaned of blood with a cotton swab and the diluting liquid is immediately drawn up to the “101” mark. The holes of the mixer are closed with the thumb and middle finger and mixed for 2-3 minutes, after which the first three drops are removed onto cotton wool, and the fourth is added to the counting chamber. If blood was drawn up to the “0.5” mark, then a 200-fold dilution is obtained, if up to “1”, a 100-fold dilution is obtained.
With the test tube method of diluting blood, take 4 ml of sodium chloride solution and add it to the test tube. Using a capillary from a Sali hemometer, 0.02 ml of blood is drawn and blown into a test tube, then the capillary is washed several times with the solution. The blood diluted 200 times is thoroughly mixed.
Stage 2
. The chamber is positioned horizontally and filled so that the entire surface on which the mesh is applied is filled with liquid. There should be no formation of air bubbles. After filling, the chamber is left strictly in a horizontal position for 1 minute to allow red blood cells to settle to the bottom.
Stage 3
. The counting of red blood cells is carried out under a microscope, preferably at medium magnification (x40 objective, x7 eyepiece) with a slightly darkened field so that the grid lines are better visible. Cells are counted in five large squares, divided into 16 small ones. Start counting from the top left large square of the grid. Then they move to the next square located diagonally, then similarly to the next one, etc. There is another principle of counting - in 4 squares in the corners and in 1 square in the center of the grid.
Counting cells in a large square begins with the upper left small square. Then move on to the second, third and fourth squares of the same row. Having counted the cells of the first row, move to the second and count in reverse order. In this way, red blood cells are counted in all 16 small squares and move on to the next large square.
To avoid re-counting red blood cells in a large square, there are two rules for counting them:
1) all red blood cells lying inside the square and on its left and upper lines are counted. Cells lying on the right and bottom lines are counted with other squares. In the rightmost squares of both the top and bottom, the cells lying on the right and bottom lines are counted with the last squares. This method involves counting only those cells that lie inside the squares, on the lines and adjacent to them on the inside. Red blood cells lying outside the square are not taken into account.
2) the second method involves counting in the same order, but differs from the first in that cells adjacent to the lines not only from the inside, but also from the outside are taken into account. The counting of red blood cells is carried out without taking into account those adjacent to the right and lower lines. According to most researchers, the first method is more accurate.
Stage 4
. The absolute number of red blood cells is calculated using the formula: X = A.B/C.G, where
X is the number of red blood cells in 1 μl of blood;
A - the number of red blood cells counted in 5 large squares;
B — degree of blood dilution (200);
B - the number of small squares in 5 large squares (80);
G is the volume of the counting chamber above the small one (1/4000 mm3).
Thus, the formula looks like: X = A.10000. For example, in 5 squares the number of red blood cells is 590, then in absolute units we get 5,900,000 cells in 1 μl of blood. To recalculate the number of cells in 1 liter (SI systemic units), the result must be multiplied by 106, i.e. 5,900,000.106= 5.9.1012/l.
Counting red blood cells using electronic automatic counters. These methods are becoming increasingly widespread because they allow automation of research, increase its accuracy and eliminate subjectivity. Various conductometric meters are used, of which the most famous types are Coulter (France), Celloscope (Sweden), Pikoskel (Hungary), etc. The operating principle of such meters is based on the difference in electrical conductivity of the formed elements of blood and the liquid in which they are located. In this case, the cells, passing through the micro-hole of the capillary tube, change the resistance of the electrical circuit, which is recorded by an electromagnetic device. The number of red blood cells is displayed on the digital display after 15-30 s.
The blood of healthy animals and poultry contains the following number of red blood cells: cattle - 5.0-7.5; sheep - 7.0-12.0; goats - 12-18; horses - 6.0-9.0; pigs - 6.0-7.5; dogs - 5.2-8.4; chickens - 3.0-4.0 per 1012/l.
A decrease in the number of red blood cells (erythrocytopenia, oligocythemia) is most often observed in anemia of various origins (posthemorrhagic, hemolytic, iron and vitamin deficiency, hypo- and aplastic, which are associated with impaired hematopoiesis). Erythrocytopenia also develops with infectious anemia of horses, hematuria of cattle, and with many acute and chronic intoxications.
An increased content of red blood cells in the blood - erythrocytosis (polycythemia) is observed more often in diseases associated with loss of fluid by the body, in particular in neonatal dyspeptic and diarrheal syndromes. It occurs in the initial stage of infectious and febrile diseases, with abdominal pain syndrome, with heart defects in the stage of decompensation, with phosphorus, mercury, carbon monoxide poisoning, and alveolar emphysema.
Lecture No. 5
Topic: STUDY OF THE BLOOD SYSTEM
Lecture outline:
1. Calculation of red blood indices and their significance in the differential diagnosis of anemia.
2. Anemic and polycythemic clinical and laboratory syndromes.
3. Methods and clinical significance of determining the number of leukocytes.
4. Indications for the study and methods for counting platelet counts.
1. Calculation of red blood indices and their meaning
in the differential diagnosis of anemia
In a number of diseases, a change in the saturation of erythrocytes with hemoglobin may occur, which is determined by calculating red blood indices - the color index (CI) and the hemoglobin content in the erythrocyte. CP gives an idea of the ratio of hemoglobin concentration to the number of red blood cells. The determination method is based on comparison of the results obtained with standard indicators of healthy animals.
CP is calculated using the formula: CP = Hb2.E1/Hb1.E2, where hemoglobin 1 and red blood cells 1 are the average values for a healthy animal of a given species and age; hemoglobin 2 and red blood cells 2 are the indicators found in the animal under study. For example:
In all healthy animals, the CP is 1+0.15, i.e. the range of fluctuations is from 0.85 to 1.15.
To determine the average saturation of red blood cells with hemoglobin, another index is calculated: SGE. In this case, they are guided by the formula:
SGE = Hb (g/l):E (1012/l).
SGE is equal to: in cattle - 15-20; pigs - 16-19; horses 17-20 pg.
Determination of red blood indices is important in the differential diagnosis of anemia. In subacute posthemorrhagic anemia, when the hemoglobin content and the number of red blood cells simultaneously decrease, the CP approaches 1, and the SGE is the same as in healthy animals. CP is less than one and a decrease in SGE, or hypochromia, occurs with nutritional, as well as with chronic posthemorrhagic anemia. For these nosological forms of anemia, the symptom of hypochromia is typical. Hyperchromia, when the CP is greater than one and the SGE increases, is a characteristic sign of hemolytic anemia, since the number of red blood cells decreases significantly.
2. Anemic and polycythemic clinical and laboratory syndromes
In the internal pathology of animals, the following main groups of anemia are distinguished: 1) posthemorrhagic (after blood loss); 2) hemolytic (due to increased destruction of red blood cells); 3) hypo- and aplastic (associated with hematopoietic disorders); 4) iron and vitamin deficiency, or nutritional (due to deficiency of iron, vitamin B12 and folic acid).
Anemia is a clinical and hematological syndrome characterized by a decrease in hemoglobin concentration, more often with a decrease in the number of red blood cells per unit volume of blood. A characteristic feature of true anemia is either a functional insufficiency of the erythrocyte system due to a reduced hemoglobin content in each cell, or an absolute decrease in the erythrocyte mass (hypovolemia).
Anemia is almost always secondary and is a manifestation of some disease. The pathogenetic mechanism of anemia can be any disease that leads to a decrease in the amount of blood in the body as a result of either acute or chronic blood loss, or increased destruction of blood (hemolysis), or impaired formation of red blood cells in the bone marrow. In many diseases and pathological conditions, anemia, its depth and nature is the leading syndrome and determines the prognosis of the disease.
Symptoms of anemia: pallor of the mucous membranes and skin; dyspnea; tachycardia with pounding heartbeat; hypochromemia (oligochromemia), erythrocytopenia (oligocythemia), increased ESR. With severe anemia, collapse may develop. Severe hemolytic anemia is manifested by hemoglobinuria.
Anemic syndrome in animals manifests itself in the following pathological conditions and diseases: external and internal, acute and chronic bleeding; poisoning with hemolytic poisons and intoxication; hemosporidiosis; deficiency or impaired absorption of iron, hypovitaminosis; erythropoiesis disorders; autoimmune and infectious diseases with hemorrhagic syndrome (see), radiation sickness. Anemia also develops when animals are not fed enough.
Polycythaemic syndrome is a pathological condition characterized by an increase in the number of formed elements per unit volume of blood. Since the majority of the mass of formed elements is erythrocytes, this condition is often called erythremia, although this term is valid only in relation to systemic absolute and primary erythrocytosis caused by the pathology of the erythroid germ of bone marrow hematopoiesis. Absolute erythrocytosis is caused by hypoxia, pulmonary artery stenosis, and methemoglobinemia. Relative (temporary) erythrocytosis is associated with loss of body fluid, stress, systemic arterial hypertension, and increased physical activity.
Symptoms of polycythaemic syndrome: dark cherry coloration of the mucous membranes, hemorrhages; erythro- and lymphocytosis; slowing down of ESR (virtually absent in cattle); hepatomegaly in cattle, splenomegaly in horses.
Polycythaemic syndrome develops with loss of water due to diarrhea, hyperhidrosis, the formation of transudates and exudates; for pulmonary failure due to bacterial, viral and parasitic pneumonia, chronic alveolar emphysema; with decompensated heart failure. The syndrome can develop in diseases with obstruction of the gastrointestinal tract (gastric dilatation, flatulence and intestinal enteralgia, ileus). Polycythemia is typical of equine infectious encephalomyelitis.
3. Methods and clinical significance of determining the number of leukocytes
Leukocytes, or white blood cells, primarily perform a protective function in the body. Depending on their forms, they participate in phagocytosis, the production of interferon, lysozyme, preperdin, histamine and other biologically active substances. Lymphocytes play a major role in specific protective reactions - the formation of cellular and humoral immunity.
Two methods have been proposed for counting leukocytes:
1. Counting in a counting chamber (method error +7%).
2. Counting in automatic counters (error +2%).
When counting leukocytes, the same chamber with a Goryaev grid is used as for red blood cells. Blood is diluted with Turk's liquid in a melangeur or in a test tube. Composition of Turk's liquid: 100 ml of 3% acetic acid solution and 1 ml of 1% gentian violet or methylene blue solution. Its purpose is to destroy red blood cells and stain white blood cells.
When diluting in a melanger, draw blood into a mixer for leukocytes to the “0.5” or “1” mark, and Turk’s liquid to the “11” mark and mix for 2-3 minutes. Receive a dilution of 10 or 20 times, respectively. For dilution using the test tube method, 0.4 ml of Turk's liquid is measured into a test tube and 0.02 ml of blood is added to it, which is drawn with a Sali capillary. The contents of the test tube are thoroughly mixed.
Filling the counting chamber is carried out in the same way as when counting red blood cells. After 1-2 minutes, after leukocytes settle to the bottom of the chamber, leukocytes are counted in 100 large, unmarked squares. The absolute number of cells is calculated using the formula:
X = A.B/C.G, where
X is the number of leukocytes in 1 μl of blood;
A - the number of leukocytes counted in 100 large squares;
B — degree of blood dilution (20);
B is the number of small squares in 100 large squares (1600);
G is the volume of the counting chamber above the small one (1/4000 mm3).
Thus, the formula looks like: X = A*50. For example, 180 leukocytes are counted, then in absolute units we get 9,000 cells in 1 μl of blood. To recalculate the number of cells in 1 liter (SI systemic units), the result must be multiplied by 106, i.e. 9,000*106= 9*109/l.
The principle of counting leukocytes using automatic counters is the same as when determining the number of red blood cells.
The blood of healthy animals and poultry contains the following number of leukocytes: cattle - 4.5-12.0; sheep - 6.0-14.0; goats - 8.0-17.0; horses - 7.0-12.0; pigs - 8.0-16.0; dogs - 8.5-10.5; chickens - 20.0-40.0 per 109/l.
An increase in the number of leukocytes in the blood - leukocytosis - can be physiological, drug-induced and pathological. Physiological leukocytosis occurs during pregnancy, after physical exertion, after eating carnivorous food, and under stress. Drug-induced leukocytosis is observed after parenteral administration of protein preparations, vaccines, serums, alkaloids, etc. to animals.
Pathological leukocytosis is observed in purulent-inflammatory processes that accompany a number of internal diseases: bronchopneumonia, pneumonia, pleurisy, pericarditis, reticuloperitonitis, etc. Severe leukocytosis is observed in many infectious diseases, leukemia, and surgical infection. It also occurs when animals are poisoned with mercury, arsenic, or an overdose of camphor.
A decrease in the number of leukocytes - leukcytopenia - is the result of inhibition of the hematopoietic organs, their exhaustion, and decreased reactivity of the body. Leukocytopenia develops as a result of infectious diseases (classical swine fever, infectious equine encephalomyelitis, salmonellosis, stachybotriotoxicosis, etc.), radiation injuries, and drug overdose (sulfonamides, levomecithin, synthomycin, etc.). Detection of leukocytopenia in diseases characterized by leukocytosis indicates a reduced natural resistance of the body and a severe course of the disease.
Goryaev's camera. Practical use
Laboratory chamber Goryaev , named after the Russian doctor, professor of Kazan University Goryaev N.K.
, is a special monolithic glass slide designed to count the number of cells in a given volume of liquid. In addition, using the Goryaev camera you can determine the magnification of the microscope.
Goryaev cameras are widely used in the field of clinical and biomedical research.
Popular areas of application of the Goryaev camera:
- Blood cell counting Red blood cell counting
- White blood cell count
- Reticulocyte count
- And so on.
Goryaev cameras are available in two modifications: two-grid (two-chamber) and four-grid (four-chamber). In determining the price of a Goryaev camera, an important role is played by the quality of glass grinding and the method of applying the mesh - laser engraving or vacuum deposition.
What is Goryaev's camera? Goryaev's camera is nothing more than a transparent monolithic glass slide with transverse slits and a specially applied microscopic mesh.
In the case of Goryaev’s two-chamber chamber, we have four slots forming three transversely located platforms, while the middle platform is divided by a longitudinal slot into two identical chambers, on each of the surfaces of which a grid is applied.
In the case of Goryaev’s four-chamber chamber, we obtain a glass slide with five slits, forming four platforms, while the two internal ones are additionally divided by a longitudinal slot to obtain four chambers with a microscopic mesh applied on the surface of the platforms.
Let's take a closer look at the features of the grid . A special mesh is applied to the internal pads located 0.1mm below the adjacent side pads. The side platforms are designed for grinding the cover glass until Newtonian rings appear.
As a rule, a special cover glass for the Goryaev chamber with rounded edges is used. After rubbing the cover glass, a chamber is created, closed on two sides, and on the other two there are gaps (the so-called capillary spaces), through which the chamber is filled.
What exactly is a grid? The microscopic grid of Goryaev's chamber is divided into large and small squares, grouped in various ways.
Goryaev's grid contains 225 large squares (15 rows of 15 large squares each), graphed vertically, horizontally, crosswise and not graphed. In this case, the size of small divisions of the grid cell is 0.05 mm, and large divisions are 0.2 mm. It is important that the small square has side 0.
05mm is a constant value in all grids. It is not difficult to calculate that the area of a small square is 0.0025 mm2, and that of a large square is 0.04 mm2. Then we find that the volume of liquid above the square formed by large divisions of the Goryaev grid is 0.004 microliters.
By counting the number of formed elements (FE) over a large square, you can calculate the density of a given cell type in suspension using the formula:
X=M*2.5*105
where X is the number of PE/ml, M is the number of PE over the large square.
When working with the Goryaev camera, it is important to ensure that its working surfaces remain dry and clean. In addition, when counting shaped elements, the presence of air bubbles on the chamber grid should not be allowed, as they may interfere with the accuracy of the count.
After working with Goryaev’s cameras, they should be disinfected using one of the acceptable methods:
- Immersion in 70% ethyl alcohol solution for 30 minutes
- Immersion in 4% formalin solution for 60 minutes at room temperature.
Let us give examples of the use of the Goryaev camera and some formulas.
Practical application of the Goryaev camera
Before starting laboratory tests, it is recommended to thoroughly wipe Goryaev’s chamber with a small piece of clean bandage lightly moistened with alcohol. We do not recommend using cotton wool for this purpose, as it may leave fibers. The cover glass for Goryaev’s camera should be treated in the same way.
Please note that when using low-quality alcohol, a sediment may form on surfaces, which in one way or another interferes with research. To avoid unwanted effects associated with this phenomenon, it is recommended to additionally wipe the camera and cover glass with a clean, alcohol-free gauze ball.
The rubbing of the cover slip to the chamber must be done very carefully until rainbow rings (the so-called Newton's color rings) appear at the contact site on both edges.
For better rubbing, you can use one trick and lightly exhale air onto the camera and cover glass, so that a small amount of moisture condenses on the surfaces of the glasses, which will ensure better contact.
In the absence of special cover glasses supplied with the Goryaev camera, ordinary standard cover glasses can be used.
In addition to the intended use of the Goryaev camera for counting blood cells, etc., this glass can be regarded as a kind of standard for determining the magnification of a microscope. To do this, use the following formula:
X=(p1-p2)/(a*N)
where X is the magnification of the microscope; p1 – position of the left border of the Goryaev chamber cell; p2 – position of the right border of a cell or group of cells; N – number of cells between measured boundaries; a is the cell size of Goryaev’s chamber (equal to 0.05 mm).
The Goryaev chamber is also used to count the number of cells in a culture.
To count cellular elements in liquids containing them in lower concentrations,
Fuchs-Rosenthal and Nageotte , having a greater depth - 0.2 mm and 0.5 mm, respectively.
The same cameras are used in algology for quantitative recording of phytoplankton. Often the Fuchs-Rosenthal camera is used to count the formed elements of the cerebrospinal fluid.
Unlike Goryaev's camera, the large squares of the Fuchs-Rosenthal grid are not graphed and are grouped into 16 squares, with each such group limited by triple lines.
Counting blood cells
Most often, Goryaev cameras are used specifically to determine the formed elements of blood during laboratory tests. So, to count red blood cells, blood must be diluted 200 times, leukocytes - 20 times. The amount of formed elements (FE) in 1 μl of blood is determined by the formula:
N=m*4000*s/q,
where N is the required amount of PE in 1 μl of blood; m is the number of FEs in a certain number of small squares; q is the number of small squares of the Goryaev chamber grid in which PEs were counted, s is the degree of blood dilution.
Formula for counting red blood cells
To count red blood cells, 5 large or 80 small grid squares located diagonally are used. Thus, we get the following formula:
N=m*4000*200/(5*16)=m*10000
Formula for counting leukocytes
To count white blood cells, you can use one of three methods:
1. Leukocytes are counted in 64 large (empty) squares
N=m*4000*20/(64*16)=m*78.125≈m*78
2. Leukocytes are counted throughout the grid in 169 large squares (recommended for blood samples with severe leukopenia)
N=m*4000*20/(169*16)≈m*29.6
3. Leukocytes are counted in 100 large squares (64 empty + 36 lined squares around the perimeter of the grid)
N=m*4000*20/(100*16)=m*50
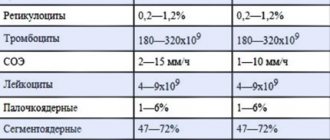
Table of normal values:
| Shaped elements | Norm | |
| Red blood cells | Men | 4,000,000 – 5,100,000 in 1 µl |
| Women | 3,700,000 – 4,700,000 in 1 µl | |
| Leukocytes | 4,000 – 9,000 in 1 µl |