Multiple myeloma (myeloma disease)
Multiple myeloma, multiple myeloma, multiple myeloma.
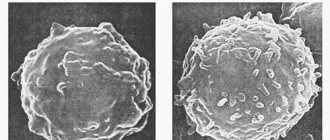
With myeloma, clinical manifestations appear that are explained by the proliferation of myeloma cells in the bone marrow and the action of the immunoglobulins and free light chains they produce. As a result of the displacement of healthy bone marrow, suppression of normal hematopoiesis is observed, which is manifested by increased fatigue due to anemia, hemostasis disorder due to thrombocytopenia, relapses of infection as a result of hypogammaglobulinemia or leukopenia. Proliferation of myeloma cells and osteoclast activity lead to hypercalcemia, the appearance of “stamped” bone defects and pathological fractures. Deposition of monoclonal immunoglobulin or free light chains causes direct kidney damage, leading to either tubular or glomerular damage (cast nephropathy or light chain deposition disease, respectively) or infiltration of various organs (heart, liver, small intestine, nerves), as in systemic AL amyloidosis. Blood hyperviscosity syndrome often develops with elevated IgA or IgM monoclonal immunoglobulin (paraprotein) and can manifest itself as cerebrovascular or respiratory failure. An increased erythrocyte sedimentation rate can be considered as a marker of monoclonal hypergammaglobulinemia and as a frequent reason for examining elderly patients.
Although some people first develop symptoms of myeloma after age 50, the average age of patients at the time of diagnosis is 66 years, and only 2% of patients are under 40 years of age. Multiple myeloma develops from an immunological condition defined as monoclonal gammopathy of unknown significance (MGUS). This condition, according to American statistics, can be detected in 2-4% of the population over 50 years of age. Since monoclonal gammopathy does not cause any complaints, it is defined only as an accidental laboratory finding and is a precancerous condition. The transition from monoclonal gammopathy of unknown significance to myeloma within a year is observed in one in 100 people affected by MGUS. Such transformation is usually observed through the intermediate stage of smoldering multipe myeloma (SMM), at which the risk of progression increases 10 times, i.e. up to 10% per year. Against the background of smoldering myeloma, there is a sharp increase in the content of paraprotein in the blood, reaching the level of advanced myeloma.
Chemotherapy treatment
If calcium levels are dangerously elevated, amyloidosis has led to kidney failure, bone marrow hematopoiesis is depressed, bones are painful and have multiple lesions and pathological fractures, there is concern that pressure is being placed on the spinal cord. In this case, chemotherapy is necessary. It can be carried out in several modes:
- In the simplest version, monochemotherapy is prescribed with alternating courses of cytostatics. The drugs used are Melphalan and Cyclophosphamide. The first is taken in low doses (0.5 mg/kg) orally for 4 days with a break of 4-6 weeks and alternates with regular intravenous infusions of 16-20 mg/m2 every 2 weeks. Cyclophosphamide is administered intramuscularly at 150-200 mg per day.
- For patients with a worse prognosis, polychemotherapy is performed. For this, not only Melphalan and Cyclophosphamide are used, but also Prednisolone, Vincristine, Doxorubicin or Dexamethasone. However, there is an opinion that, despite its greater intensity, such a program does not increase either the number and duration of remissions or survival.
- During remission after chemotherapy, alpha interferon drugs can be used. When administered intramuscularly at a dose of 3-6 million units three times a week, the period of improvement is extended. Interferon is effective both in combination with other drugs and on its own.
- In cases where people under the age of 65 years are ill, high-dose chemotherapy with Melphalan is mainly used, followed by transplantation of a donor or their own bone marrow. This makes it possible to increase disease-free and overall survival with a high degree of efficiency.
A potential cure can only occur with a successful donor bone marrow transplant. However, this method is limited by the age of the patient and the high probability of mortality (5-10%) from the toxic effects of the treatment itself. Therefore, usually only monitoring the course of the disease and ensuring long-term remission remains available.
2014 Multiple Myeloma Classification
In 2014, the International Multiple Myeloma Working Group updated the diagnostic criteria for various forms of this disease. The major change was the addition of three specific biomarkers to the existing markers of end organ damage (hypercalcemia, renal failure, anemia, or bone damage): clonal bone marrow plasma cell count ≥60%, serum free light chain ratio ≥100, and more than one localized lesion on MRI. Previously, end organ damage was interpreted as the acronym CRAB - calcium, renal disease, anemia, bone lesions.
The updated criteria allow for early diagnosis and treatment before end-organ damage develops. As per the criteria, the diagnosis of multiple myeloma requires the presence of 10% or more plasma cells on bone marrow examination or the presence of biopsy-proven plasmacytoma, plus one or more disease-related abnormalities.
International Working Group Diagnostic Criteria for Multiple Myeloma and Related Cellular Disorders (2014)
- Monoclonal gammopathy of unknown significance - MGUS: monoclonal paraprotein (non-IgM) - <30 g/l, clonal plasma cells in bone marrow <10%, no manifestations of end organ damage such as hypercalcemia, renal failure, anemia and damage bones, which may be associated with plasma cell proliferation.
- Smoldering multiple myeloma (Smoldering MM) : serum monoclonal protein (IgG or IgA) ≥30 g/L, or urine monoclonal protein ≥500 mg/24 hours and/or clonal plasma cells in bone marrow 10%-60%, no myeloma-related complications or amyloidosis.
- Multiple myeloma: Clonal bone marrow plasma cells - ≥10% or biopsy-proven bone or extramedullary plasmacytomas. To make a diagnosis, one or more of the following myeloma-related complications (MDE-myeloma defining event) must be identified:
- Hypercalcemia: serum calcium >0.25 mmol/L or higher than the upper limit of normal for the appropriate laboratory value or >2.75 mmol/L;
- Renal failure: creatinine clearance <40 ml/min or serum creatinine >177 μmol/L;
- Anemia: hemoglobin value >20 g/L below the lower limit of normal in the laboratory or hemoglobin value <100 g/L;
- Bone damage: one or more osteolytic lesions on skeletal radiography, computed tomography, or positron emission tomography;
- The percentage of clonal plasma cells ≥60% : as an independent finding is interpreted as a condition sufficient to make a diagnosis (MDE-myeloma defining event);
- Ratio of involved/uninvolved free light chains ≥100 : provided that the concentration of involved light chains exceeds ≥100 mg/L;
- More than one localized bone lesion at least 5 mm in length on MRI.
- Monoclonal gammopathy of unknown significance with IgM paraprotein (IgM-MGUS) : all 3 criteria must be present: monoclonal IgM protein <30 g/l, lymphoplasmacytic bone marrow infiltration <10%, no signs of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy, hepatosplenomegaly , which may be attributed to lymphoproliferative disorder (Waldenström's disease).
Stages of the disease
- Stage I. The amount of hemoglobin is more than one hundred, the level of calcium in the blood is within normal limits, there is no bone destruction. Immunoglobulin J is less than 50 g/l, immunoglobulin A is less than 30 g/l. Bence-Johnska protein in urine is less than 4 grams per day.
- Stage II. The amount of hemoglobin is from 85 g/l to 120 g/l. Moderate bone destruction. Immunoglobulin J 50 – 70 g/l, immunoglobulin A 30 – 50 g/l. Bence Jones protein 4 – 12 grams per day.
- Stage III. The amount of hemoglobin is less than 85 g/l. The level of calcium in the blood exceeds normal levels. Noticeable bone destruction. Immunoglobulin G is above 70 g/l, immunoglobulin A is more than 50 g/l. Bence Jones protein in urine is more than 12 grams per day.
By degree of progression:
- smoldering – the disease does not progress over many months and years;
- slowly progressive;
- rapidly progressing;
- aggressive.
Diagnosis of multiple myeloma
Laboratory diagnosis and screening of paraproteinemias is based on the identification of blood “paraprotein”. A very sensitive method for diagnosing paraproteinemia is immunofixation of serum and urine proteins with a panel of IgG, IgM, IgA, IgE, IgD, kappa, lambda antisera. The diagnostic significance of detecting paraproteinemia increases significantly with a characteristic clinical picture indicating a plasma cell disease. When screening asymptomatic individuals by electrophoresis or immunofixation in the absence of clinical manifestations of myeloma, the detection of a paraprotein indicates monoclonal gammopathy of undetermined significance (MGUS). Clinical indications for paraprotein testing include bone pain, pathological fractures, polyneuropathy, fever, and anemia. Paraproteinemia is characterized by such laboratory findings as an increase in erythrocyte sedimentation rate, proteinuria and azotemia, hypercalcemia, an increase in the content of total serum protein, as well as deviations from the norm in the content of the main protein fractions. Infectious processes also often accompany myeloma, since paraprotein synthesis suppresses the synthesis of normal immunoglobulins, which leads to dysfunction of the immune system. Immunochemical study of the main serum immunoglobulins IgG, IgA, IgM allows us to identify changes in their synthesis. However, when assessing paraproteinemias, immunochemical determination of immunoglobulins is not recommended due to the frequent phenomenon of “prozone” at high concentrations and the inaccuracy of measuring monoclonal molecules, since the characteristics of synthesis in the myeloma cell change the antigenic properties of immunoglobulins. In all these cases, the method of choice for detecting and measuring paraprotein is electrophoresis with immunofixation of serum and urine proteins. The paraprotein is represented by immunoglobulin IgG in approximately half of the patients, IgA in 20%, IgD in 2%, IgM in 0.5%. In 20% of patients, the paraprotein is represented only by free immunoglobulin chains. In 2-3% of cases of the disease, the paraprotein is not detected, which is considered as non-secreting myeloma. To be precise, this name is misleading because in nonsecreting myeloma, free light chains can be found in serum or urine. A diagnostic panel consisting of a combination of serum protein electrophoresis, serum immunofixation and determination of free light chains in serum or electrophoresis with immunofixation in a 24-hour urine sample is recommended for all persons with suspected multiple myeloma. Sensitivity for detection of monoclonal proteins averaged 82% for protein electrophoresis, 93% for immunofixation, and 97% when adding free light chain or protein electrophoresis and immunofixation results in a 24-hour urine sample. The absence of a monoclonal protein in approximately 2% of patients is typical of nonsecretory myeloma.
In the vast majority of cases, the diagnosis of myeloma begins after the appearance of characteristic symptoms. The diagnosis of multiple myeloma after the appearance of symptoms such as increased fatigue and back pain in practice is usually delayed by more than 3 months. Although it is unknown how this affects the overall outcome of the disease, the incidence of complications and hospitalizations during this delay period increases, which negatively affects the quality of life of patients. Many factors influence the cause of the delay, including the nonspecific nature of complaints and disorders that are often observed in older people and which are initially considered benign by them and their relatives. But the persistent nature of pain in the spine and increased fatigue should always alert practitioners. Testing for musculoskeletal pain, anemia, thrombocytopenia, renal failure, hypercalcemia, and neurological disorders may result in the detection of moclonal protein in serum or urine.
In addition, the diagnostic search for multiple myeloma includes a complete count of blood cells and ESR, measurement of serum calcium and creatinine levels, electrophoresis of serum and urine proteins with immunofixation, study of free light chains in the blood, and examination of bone marrow puncture. Additionally, low-dose whole-body CT or 18-fluorodeoxyglucose PET/CT or, at a minimum, plain skeletal radiography is required to identify osteolytic bone lesions.
Bone damage
Osteolytic bone destruction is a central manifestation of multiple myeloma and is present in up to 80% of patients at the time of diagnosis. Generalized bone destruction leads to hypercalcemia, accelerated osteoporosis, the formation of kyphosis, and wedge-shaped vertebral fractures. Persistent debilitating bone pain is the leading complaint that forces patients to see a doctor for the first time. Regular affected areas include the spine and pelvic bones, which results in various types of fractures and possible compression of the spinal cord.
It has been proven that the interaction of myeloma cells with the microenvironment in the bone marrow leads to the production of cytokines, causing high activity of osteoclasts and reduced activity of osteoblasts. Therefore, there are no signs of bone repair at the sites of destruction. The use of bisphosphonates, radiotherapy, balloon kyphoplasty and reconstructive surgery are the main means of rehabilitation of patients with bone destruction. However, bone destruction persists even after the active stage of the disease is cured. To identify foci of osteolysis in the bones of the skeleton, I use various imaging tools, from radiography of all bones and low-dose computed tomography to MRI and PET of the whole body. The presence of more than one focus of lysis in bones larger than 5 mm is considered an indication for therapy to prevent further destruction in the absence of pain.
Mandatory therapy for destruction and pain in myeloma is currently the prescription of bisphosphonates. These drugs were originally proposed for the treatment of osteoporosis. But they not only reduce pain, strengthen bones, but also slow down the progression of myeloma. Due to their excretion through the kidneys and prolonged retention in the body, bisphosphonates can cause severe kidney damage, colds, and hypocalcemia. Therefore, monitoring of renal function as well as electrolytes (calcium and phosphorus) is required during intravenous bisphosphonate therapy.
Symptoms
Symptoms of myeloid leukemia are:
- Bone pain. The hip bones, spine, pelvis, ribs hurt;
Pain in bones and spine
- Pathological fractures;
- Hypercalcemia. Manifested by vomiting, nausea, constipation, polyuria. Brain disturbances may occur, the person falls into lethargy or coma;
- Kidney diseases. Nephropathy manifests itself as an increase in calcium and uric acid in the blood, the appearance of protein in the urine;
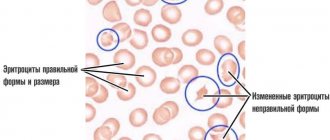
- The anemia is normochromic. The blood color index is normal, the ESR increases sharply;
- Osteoporosis;
- Compression of the spinal cord by spinal tumors. Manifests itself in the form of back pain, aggravated by coughing and sneezing. The functioning of the bladder and intestines is disrupted;
- Vulnerability to bacterial infections. Associated with weakened immunity;
- Hemorrhages. Bleeding from the nose, uterus, gums, subcutaneous hemorrhages.
Anemia
Anemia is a manifestation of myeloma in approximately 75% of patients. The anemia is usually normochromic and normocytic, with signs of hypoproliferation (reticulocyte index <2.5%), with elevated ferritin levels (an indicator of inflammation). Hypochromic red blood cell counts >5% and low transferrin saturation are typical of iron deficiency. In these cases, the level of anemia is moderate. But 10% of patients with Hb<80 g/l experience a decrease in quality of life and an unfavorable prognosis. Anemia is rarely found in individuals with initial disease. The hemoglobin level determines the time of initiation of treatment for anemia in multiple myeloma. Several factors are responsible for the development of anemia. These are infiltration of the bone marrow by myeloma cells, leading to a decrease in the number of erythroid progenitor cells, erythropoietin deficiency in patients with renal failure, decreased response of proerythroblasts to kidney erythropoietin, impaired iron utilization due to high levels of hepcidin in chronic inflammation, increased plasma volume with increased levels of paraproteins, side effect of therapy. However, the main cause of anemia in myeloma disease is myeloma cell-induced apoptosis of erythroblasts.
In case of persistent symptomatic anemia and a hemoglobin level of less than 100 g/l, the possibility of other causes of anemia (Fe-deficiency, B12-deficiency, hemolytic, chronic infections, etc.) should be excluded. In the case of iron deficiency anemia, which is determined by the number of hypochromic red blood cells of 5% and a reduced level of transferrin saturation (TLS) of less than 20%, intravenous iron supplements are used.
The hemoglobin level determines the time of initiation of treatment for anemia in multiple myeloma. One of the methods for predicting the effect of erythropoiesis-stimulating agents, in particular erythropoietins, is to determine the integrity of bone marrow function. Since thrombomodulin, which stimulates thrombocytosis, is synthesized mainly by the liver, the bone marrow resource is preserved when the number of platelets in the blood is more than 150x10^9 cells/l. An initially reduced level of erythropoietin in the blood is important for predicting a positive response to recombinant erythropoietin therapy, which makes it possible to avoid red blood cell transfusions. Frequent side effects of the use of erythropoietins are thromboembolic complications and arterial hypertension.
Other methods
If there is only one focus of plasma cell proliferation, surgical treatment can be performed. It is also indicated as an addition to chemotherapy for signs of compression of vital organs, especially the spinal cord. Surgical treatment in this case consists of removing pathological formations or even part of the vertebral bone. Additionally, glucocorticoids and radiation therapy are prescribed.
The latter improves the quality of life of weakened patients and is usually prescribed in combination with other methods. It is mandatory in case of renal failure and resistance to chemotherapy. As the primary treatment, radiation therapy is used for localized bone lesions.
Additionally, symptomatic treatment should be carried out, consisting of the administration of fluids and correction of hypercalcemia, as well as taking analgesics, hemostatic therapy and orthopedic care. Pamidronate, zoledronic acid, or other bisphosphonates are commonly used as drugs to stop bone destruction and prevent fractures . In some cases of anemia, erythropoietin administration and red blood cell transfusions are required.
Kidney damage.
There are several mechanisms of kidney damage in multiple myeloma. Mild renal failure, assessed as a decrease in GFR below 60 ml/min/1.73 m2, is found in 20% of myeloma patients at diagnosis and in approximately half of patients during the course of myeloma disease. The causes of kidney damage are complex and include dehydration, hypercalcemia, infections, exposure to nephrotoxic drugs, in particular, taking large doses of NSAIDs to relieve pain.
Most characteristic of myeloma is the finding of tubulointestinal kidney damage, known as cast nephropathy, which is a direct consequence of increased levels of free immunoglobulin light chains in the blood. Tubular epithelial cells are damaged and atrophy due to increased “transfer” of free chains from the lumen of the tubules to the interstitium. Against the background of dehydration, acute renal failure syndrome may develop due to impaired blood flow and tubular disorders.
The presence of light chains in the urine of multiple myeloma patients can cause kidney dysfunction known as secondary Fanconi syndrome. It is caused by insufficient reabsorption capacity of the proximal tubules, which is manifested by glucosuria, aminoaciduria, hypophosphatemia and hypouricemia.
In the interstitium, an inflammatory process develops with resulting tubulointerstitial fibrosis, leading to renal failure. Moreover, monoclonal light chains that are not excreted by the kidneys can be deposited in the kidneys, heart, liver, small intestine, and nerve trunks, leading to the development of primary amyloidosis (AL amyloid) or light-chain deposition disease (LCDD). . Diagnosis of renal failure requires determination of creatinine, urea, sodium and potassium, calcium and assessment of GFR using the MDRD or CKD-EPI formula. Measurement of total protein, electrophoresis, and immunofixation in 24-hour urine samples is also recommended. In patients with nonselective proteinuria or selective albuminuria, it is necessary to exclude the presence of amyloidosis or MIDD, for which a biopsy of the kidney or subcutaneous fat with Congo-oral staining is indicated. In patients with free light chain proteinuria (Bence Jones protein), a fat biopsy is not necessary, since in this case the diagnosis of myeloma kidney damage is not in doubt and requires planning of treatment for the underlying disease.
Free light chains have high sensitivity and specificity compared to urine protein electrophoresis. Patients with renal failure due to MM have an elevated kappa/lambda ratio even in the absence of evidence of monoclonal gammopathy. The reason is a violation of the release of light chains. In healthy people, the blood is cleared of light chains by the kidneys. Kappa chains are monomeric and leave the blood faster than lambda chains, as evidenced by an average kappa/lambda ratio of 0.6 in individuals without kidney damage. In patients with renal failure, the reticuloendothelial system becomes the main purification system; the half-life of the kappa chains is extended because of this. The kappa/lambda ratio in renal failure is on average 1.8. Free light chains have high sensitivity and specificity, and patients with renal failure have an increased kappa/lambda ratio due to impaired clearance of kappa and lambda chains.
Infectious complications in multiple myeloma.
In multiple myeloma, the frequency of bacterial and viral infections increases by 7-10 times compared to population controls. Haemophilus influenzae, streptococcus pneumoniae, Escherichia coli, gram-negative bacteria and viruses (influenza and herpes zoster) are the most common culprits of infection in patients with multiple myeloma.
The increased sensitivity of patients to infectious diseases is the result of two main circumstances. Firstly, the influence of the disease itself, and secondly, old age and the side effects of the therapy. Lymphocytopenia, hypogammaglobulinemia, neutropenia due to infiltration of myeloma cells in the bone marrow and under the influence of chemotherapy cause increased sensitivity to infection. Disease-associated innate immune deficiency involves different parts of the immune system and includes B cell dysfunction as well as functional abnormalities of dendritic cells, T cells, and natural killer (NK) cells. Impaired kidney and lung function, gastrointestinal mucosa, and multiorgan disorders caused by the deposition of immunoglobulin light chains also increase the risk of infectious diseases. Finally, multiple myeloma predominantly affects older people with comorbid age-related diseases and a sedentary lifestyle, who are initially predisposed to infections.
Immunomodulators and glucocorticoids are part of the treatment for the most severe variants of the disease. In case of existing infectious contacts, the presence of neutropenia and hypogammaglobulinemia and suppressed cellular immunity, therapy with immunomodulators requires prophylactic antibiotics.
Additional Research
Other malignant tumors have similar symptoms to myeloma: osteomyelitis and lymphoplasmacytic lymphoma. Additional research can help rule them out.
Bone metastases can occur with malignant hemangioma, lymphoid tissue disease, cancer of the sympathetic nervous system, or breast cancer. These diseases are distinguished from myeloma by the presence of a primary tumor focus, and the morphology is confirmed by histological examination.
With osteomyelitis, tubular bones are affected, and an inflammatory reaction occurs in the blood, so pus is detected during a biopsy. In addition, this disease, unlike myeloma, is more common in young people.
A distinctive feature of lymphoplasmacytic lymphoma, which causes enlargement of the lymph nodes and spleen, damage to the bone marrow and lungs, is the production of monoclonal protein and large polypeptide subunits of antibodies.
Hypercalcemia
Symptoms of hypercalcemia are nonspecific and depend on both the absolute values and the timing of calcium increase. Moderate hypercalcemia (serum calcium 3–3.5 mmol/l), which developed over months, can be tolerated unnoticed with minimal complaints, while hypercalcemia that occurs over a week period leads to significant symptoms. Severe hypercalcemia (calcium more than 3.5 mmol/l) almost always leads to clinical manifestations. Patients complain of lack of appetite and constipation. In this case, general malaise and muscle weakness can progress to lethargy, confusion and coma. Cardiovascular manifestations include shortening of the QT interval and arrhythmias. Renal dysfunction appears to be another important manifestation of hypercalcemia. Patients often report polyuria as a consequence of decreased renal concentrating ability in conditions of hypercalcemia. The appearance of kidney stones is observed only with prolonged hypercalcemia. Acute renal failure as a result of direct vasoconstriction and natriuresis-induced reduction in blood volume is among the most severe manifestations of renal damage due to hypercalcemia. The cause of hypercalcemia is increased activity of osteoclasts with decreased activity of osteoblasts and lysis of bone tissue in multiple myeloma. Activation of osteoclasts, which destroy bone structure, is caused by cytokines secreted by myeloma cells, in particular interleukin-1. It is no coincidence that the degree of hypercalcemia depends on the total mass of accumulated myeloma cells, so that the most severe hypercalcemia is found in patients with widespread disease.
Signs of hypercalcemia depend on calcium levels and how quickly they rise, necessitating rapid evaluation. Most causes of hypercalcemia in practice are due to increased levels of parathyroid hormone and its derivatives (humoral form), and in 20% - with infiltration of the bone marrow by tumor cells (infiltrative form). Multiple myeloma with light chains in the blood is the most common cause among blood diseases. The number of patients with hypercalcemia due to the presence of a tumor is 2-3 times higher than due to primary hyperparathyroidism.
Myeloma with hypercalcemia is characterized by low levels of parathyroid hormone, phosphorus is normal. In the humoral form of hypercalcemia, an increased level of parathyroid hormone and a low level of phosphorus are detected.
Preparing for analysis
The rules for donating blood for a general analysis do not provide for specific preparation rules. It is known how to take a blood test for chronic myeloid leukemia. Blood is donated on an empty stomach, in the morning, to avoid “interference” that distorts the results. Heavy physical activity is not recommended on the day before donating blood. It is highly undesirable to consume fatty and fried foods within three days before the procedure. If these conditions are met, then a diagnostic blood test for myeloid leukemia will be extremely informative.
Blood is drawn from a vein or finger. Venous blood is more concentrated than capillary blood, which is why some doctors require this type of sampling for analysis.
Decoding the results of myeloid leukemia takes two days from the moment the results are accepted for processing. If the laboratory is overloaded with work, the result may be obtained later.
Modern blood diagnostics involve taking bone marrow samples from the femur for cytogenetic analysis. Samples are collected by biopsy or aspiration. Chromosomes are studied. The affected cells contain an abnormal chromosome 22. To detect an abnormal chromosome, a polymerase chain reaction is used.
Thrombophilia
The risk of venous thrombosis is due to a number of reasons, and myeloma significantly increases it. Risk factors for thrombosis include advanced age, limited mobility due to pain, frequent infections, dehydration, renal failure, obesity, diabetes mellitus and other comorbid diseases.
Among the manifestations, the most dangerous is pulmonary embolism, which can be fatal.
The incidence of thromboembolism in myeloma is estimated at 5-8/100 patients.
This is due to the fact that myeloma is accompanied by increased blood viscosity, inhibition of the production of natural anticoagulants and hypercoagulation of the blood caused by infections, with increased levels of von Willebrand factor, fibrinogen and factor VIII, decreased levels of protein S, and so on. A course of drug therapy, including the prescription of erythropoietins, can also play a role as a trigger for venous thromboembolism. Therefore, in the first months of therapy, it is recommended to supplement traditional myeloma therapy with aspirin or anticoagulant therapy.
Screening for predisposition to thrombosis and venous thromboembolism in myeloma, along with a standard coagulation examination, should include a blood viscosity test.
Causes and risk factors
The reason why plasma cells become malignant has not been established. Presumably, there is a genetic predisposition. Viral infections, ionizing radiation (including radiation therapy), carcinogens, cytostatic drugs (chemotherapy), and chronic intoxication can act as mutagenic factors. In 10% of people suffering from monoclonal gammopathy, it transforms into myeloma.
Predisposing factors include everything that has a suppressive effect on the immune system: obesity, bad habits, unhealthy lifestyle, stress tolerance, etc.