Aplastic anemia is a special case of impaired red blood cell synthesis, accompanied by a drop in hemoglobin concentration as a result of abnormalities in the functioning of the bone marrow.
Unlike other forms of the disorder, for example iron deficiency, megaloblastic, the pathology is much more difficult to cure and has a worse prognosis.
The clinical picture itself is extremely dangerous and poses a great threat to the existence of the body. The process produces bleeding of various locations that is difficult to stop.
Diagnosis presents certain difficulties due to the lack of specific clinical signs if the study is carried out using routine methods.
Treatment is strictly inpatient. Its duration is indefinitely high, depending on the severity of the pathological process, the nature of its course, aggressiveness, stage, as well as the individual characteristics of the human body, the primary cause of the disorder.
Classification
There are congenital (constitutional) and acquired forms of hypoplastic anemia. Congenital hypoplastic anemias are relatively rare. Depending on the nature of the inhibition of hematopoiesis, forms are distinguished with damage to all three germs (true G. a.) or only erythropoiesis (partial G. a.). Sometimes G. a. is isolated. with a hemolytic component, but some authors believe that it represents the hypoplastic stage of paroxysmal nocturnal hemoglobinuria (see Hemolytic anemia).
Symptoms
Almost every anemia, like hypoplastic anemia, has two main problems:
- Signs of the disease. Symptoms appear gradually and go unnoticed in the first stages of pathology development. Then primary signs appear, to which only a small proportion of patients pay attention.
- People. The second problem is the people themselves. Most of them ignore minor changes in their condition and do not pay attention to the deterioration of their well-being. But if some do this only at the early stage of anemia development, then there are those who endure discomfort and pain to the last, try to self-medicate and seek help at the very last stages.
This is the main catch. The earlier anemia (anemia) is diagnosed, the greater the chances for a full recovery and restoration of the body. Late treatment leads to serious consequences and sometimes fatal outcomes.
Therefore, you should know how hypoplastic anemia begins to manifest itself, and what is important to respond to in a timely manner.
During the initial stages of development of a hematopoietic disease, a person feels:
- general weakness in the body;
- noticeable loss of appetite;
- attacks of dizziness without objective reasons;
- chills.
We agree that such symptoms often go unattended, since people attribute everything to physical or psycho-emotional fatigue. In rare cases, a person seeks help. It’s easier for him to rest a little, take some pills and wait until everything goes away on its own.
The prolonged development of the disease and its transition to a more severe condition make diagnosis difficult. But there are cases when hypoplastic anemia developed rapidly and manifested itself in the form of:
- pain in the bones;
- partial or complete loss of limb mobility;
- hemorrhagic symptoms;
- pale skin, etc.
Patients with this diagnosis note changes in the functioning of the cardiovascular system. The symptoms are as follows:
- blood pressure decreases;
- the heart tone becomes dull;
- systolic murmurs are observed;
- signs of tachycardia appear.
Another problem is that the hematopoietic system is significantly inhibited, its functions are disrupted, and the bone marrow is depleted. Against the background of such changes, concomitant pathologies can form. And this is a double-edged sword, since their treatment requires a normal state of the hematopoietic system.
The consequences of this human condition with hypoplastic anemia can be:
- infectious pathologies developing in the patient’s body, accompanied by a significant increase in body temperature;
- the occurrence of different types of bleeding (men often have nosebleeds, and women experience uterine bleeding);
- effusions of blood occurring in the serous or mucous membranes, which is caused by a deficiency of platelets.
If you notice even the mildest symptoms and changes in your usual state of health without objective reasons, it is better for prevention to consult a doctor and get examined. Even by taking simple tests once a year, you will protect yourself from a number of diseases and will be able to stop their development at an early stage. This is much simpler and more correct than treating an already established pathology.
Story
In 1888, P. Ehrlich described a disease in a young woman, in which bleeding, fever, profound anemia and leukopenia developed acutely (platelets were not counted at that time); At the autopsy there were no signs of hematopoiesis in the bone marrow, which P. Ehrlich explained by the primary inhibition of its function. The term “aplastic anemia” was first proposed by Shoffard (A. M. Chauffard, 1904). Subsequently, aplastic anemia of the Ehrlich type was identified, in which, along with steadily progressing pancytopenia, often complicated by sepsis, pronounced hemorrhages and necrotic phenomena, histologically no signs of hematopoiesis were detected in the bone marrow; the disease was observed in persons aged 18 to 20 years. A similar disease called “hemorrhagic aleukia” was described by Frank (E. Frank, 1915). With the introduction into practice in 1927 by M.I. Arinkin of puncture examination of the bone marrow, it became possible to distinguish true hypoplastic anemia from pancytopenia caused by leukemic or metastatic lesions of the bone marrow. In the domestic literature, the first descriptions of G. a. under the name “clinical-hematological syndrome” belong to G.P. Khosroev (1913). G. a. the works of XX Vlados (1937), E. A. Kost (1952), I. A. Kassirsky and G. A. Alekseev (1962), F. E. Fainstein (1965), G. S. Mukhamedzyanova are devoted to the work of XX Vlados (1937), E. A. Kost (1952), I. A. Kassirsky and G. A. Alekseev (1962), (1970) and others.
The statistics have not been studied in detail. According to statistical data from Moscow prosecturas, the frequency of G. a. was in 1928-1932. 0.009%, in 1945-1950 0.13%, in 1951 - 1956 0.25%. In the USA (according to the state of California, 1967), the incidence of G. a. was 2 cases per 1 million people, or 1: 400,000 - 1: 700,000. A pronounced dependence of the incidence on gender, age and ethnicity has not been established.
Reasons for development
Hypoplastic anemia can be caused by the negative effect of various factors; their combination not only contributes to the development of the disease, but also leads to aggravation of the pathological process.
Very often, this type of anemia occurs due to the toxic effect of certain medications that can provoke disorders in the bone marrow with further dysfunction of hematopoietic germs. Its formation does not depend on the dosage of medications or the duration of the therapeutic course.
Drugs that can cause disturbances in the hematopoietic system include:
- sulfonamides,
- antibiotics,
- antihistamines,
- tetracyclines.
Very often, the disease is diagnosed in people taking Levomycetin for a long time
Disruption of hematopoiesis is observed after a course of chemotherapy, since the toxic effect of drugs destroys not only pathological formations, but also healthy cells and tissues.
The causes of hematopoietic function disorders are also autoimmune diseases, in which the immune mechanism is aimed at suppressing not only pathogenic microorganisms, but also its own damage to bone marrow elements.
Thus, there are three groups of main causes causing disruption of the hematopoietic function of the bone marrow:
- Hereditary. Transmission of genetically modified genes, manifested by chromosomal abnormalities.
- Main. Toxic effects of chemotherapy drugs, radiation, arsenic and benzene poisoning.
- Rare. It forms when taking certain medications or when infected with a fungus.
Factors provoking the development of the disease:
- hepatitis of viral origin;
- herpes virus;
- cytomegalovirus infection;
- HIV infection.
Ionizing radiation used during X-ray examination plays an important role in the mechanism of anemia formation. Most often, this pathology occurs among workers in radiology rooms, as well as in patients who have undergone a course of radio wave therapy.
Etiology
Hypoplasia of hematopoiesis can be caused by the influence of various external factors, which are usually divided into two groups: 1) factors with an obligate myelotoxic effect, proportional to the dose - ionizing radiation, benzene and its derivatives (benzene anemia develops), antitumor drugs (chlorethylamines; phosphoramides; antimetabolites - folic acid antagonists, analogues of purines, pyrimidines, etc.; antimitotic agents - colchicine, vinca alkaloids; antibiotics - bruneomycin, rubomycin, adriamycin, carminomycin), inorganic arsenic compounds, estrogens, etc.; 2) factors with a facultative myelotoxic effect, detected only in isolated cases - antibacterial, anticonvulsant, antithyroid, antihistamines, tranquilizers (aplastic post-drug anemia develops), insecticides, etc.; direct connection with the development of G. a. with the dose and duration of use of the drug in these cases is not noted. The second group should include relatively rare cases of development of G. a. with tuberculosis, pregnancy. G. a., associated with the ingestion of overwintered grain (see Aleukia alimentary-toxic), practically does not occur.
Of the factors with a facultative myelotoxic effect, the antibiotic chloramphenicol (chloramphenicol) is the most dangerous. According to Wallerstein et al. (R. O. Wallerstein, 1969), in persons taking chloramphenicol (see), the possibility of developing G. a. 13 times higher than the general population. Less commonly described is G. a. in connection with the intake of organic arsenic compounds (mafarsen, etc.), antimalarial drugs, hydantoin derivatives, phenylbutazone, gold salts.
Hypoplastic anemia caused by exposure to various external factors is called myelotoxic anemia. In approximately 50% of cases G. a. It is not possible to identify the cause of the disease - the so-called. idiopathic form.
Etiology of constitutional G. a. (Fanconi syndrome, or anemia, partial G. a. Josephs - Diamond - Blackfan and familial G. a. Estren - Dameshek) is most likely associated with the inheritance of the gene from one of the parents in a recessive manner. In a cytogenetic study, G. E. Bloom et al. (1966) discovered various chromosomal aberrations in hematopoietic cells in patients. Cases of the disease in children born from consanguineous marriages have been described. Some authors note the similarity of Fanconi syndrome with embryopathy (see) caused by thalidomide (see).
Causes
The causes of aplastic anemia are as follows:
- The presence of external factors that have a myelotoxic effect, that is, provoke cytostatic blood clotting disorders. This includes various diseases of an infectious and viral nature, the effects of ionizing radiation, and some medications (analgin, anti-tuberculosis drugs, some types of antibiotics), as well as drugs used in chemotherapy.
- Endogenous, that is, internal, causes of aplastic anemia are the accumulation of toxic substances as a result of internal disorders and endocrine changes, for example, in the case of the development of hypothyroidism and uremia.
- Autoaggression, when a patient develops individual sensitivity to antigens and antibodies appear in the blood.
- Idiopathic forms of aplastic anemia. Distinguished in half of the patients, diagnosed if the cause of the disease could not be determined.
The main factors of aplastic anemia
At the present stage, specialists have been able to study to the greatest extent only congenital types of aplastic anemia. So, in the case of diagnosing Fanconi anemia, the reason lies in changes in paired chromosomes I and VII. In Diamond–Blackfan anemia, the genes of chromosomes I, XVI, XIX, and XIII are mutated. The effect of free radicals on the body can play a role in these processes.
Pathogenesis
The development of hypoplastic anemia can be associated either with damage to the stem cell, the parent cell for granulo-, erythro- and thrombocytopoiesis, or with a defect in its microenvironment (see Hematopoiesis), which prevents the normal functioning of this cell. The detection of chromosomal aberrations in congenital hematopoietic aberrations, the therapeutic effectiveness of bone marrow transplantation from identical twins, as well as the small number of stem cells detected when culturing the bone marrow of patients confirm the first assumption. Rarity of occurrence of G. a. under the influence of the listed optional factors speaks about the role of individual, possibly hereditary, predisposition. The participation of autoimmune mechanisms is assumed only in partial G. a., with a cut by Krantz (S. B. Krantz, 1973) and L. I. Idelson et al. (1976) discovered antibodies to erythrocyte nuclei. OK. 50% of cases of partial G. a. develops in patients suffering from benign thymoma; the reasons for this are still unclear. With G. a. There is no deficiency of hematopoietic factors. On the contrary, their content in the blood is even increased due to incomplete use of the reduced volume of erythropoietic tissue.
Hemorrhagic manifestations with G. a. are caused by impaired hemostasis as a result of deep thrombocytopenia and damage to the vascular wall. Increased permeability of the vascular wall is secondary and is associated with hypoxia and a lack of serotonin. Histochemical, and immunomorphological l. studies reveal deep structural disorders in the vascular wall.
Iron-deficiency anemia
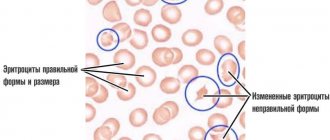
Based on the name of the pathology, it becomes clear what occurs as a result of an acute lack of this element in the human body. The reason why iron deficiency pathology develops may be prolonged blood loss (menstrual flow, ulcerative lesions, etc.). Often, diagnostics of pregnant girls reveals the presence of such a disease due to the high need for microelements at this stage of life. Iron deficiency, when examined in the laboratory, shows low hemoglobin, as well as red blood cells and iron in small quantities. Iron deficiency is treated by using medications with a high iron content, either orally or in the form of injections. Deciphering for IDA will show a violation of the morphological features of the erythrocyte and their index. It also differs in anisocytosis - when the red blood cells of one person differ in size from each other. In addition, the pathology is characterized by poikilocytosis - a difference in the shape of the red blood cells of one patient.
Progressive iron deficiency during a biochemical examination of the blood reveals quite low serum ferritin, low amounts of iron, increased TCV, transferrin is poorly saturated with iron. Iron deficiency has three stages of development: mild, moderate, severe. With such a mild form of the disease, hemoglobin is in a low position, but above 88 g/l. Moderate form of hemoglobin keeps at around 72 g/l. With severe development, it reduces the hemoglobin concentration below 69 g/.
Pathological anatomy
At autopsy, signs of anemia and dystrophy of internal organs are found, abundant fat deposition in the subcutaneous tissue, epicardium, omentum, multiple hemorrhages in the skin, mucous membranes, serous tissues, and internal organs. Sometimes massive hemorrhages in the brain or heart muscle are noted, which are the direct cause of death. The bone marrow of flat bones is pale pink or yellowish in color, sometimes with dark red areas of hemorrhage. When the bone is compressed, a bloody fluid rich in fat drains from the cut surface. Areas of preserved hematopoietic tissue may be observed among the fatty bone marrow. The size of the spleen and lymph nodes is often reduced.
Rice. 1. Microscopic specimen of the liver (hypoplastic anemia): the arrow indicates the deposition of hemosiderin. Rice. 2. Microscopic specimen of a kidney (hypoplastic anemia): arrows indicate the deposition of hemosiderin in the tubular epithelium.
Microscopic examination of the bone marrow (see) reveals varying degrees of its devastation. In the case of aplasia, only small accumulations of lymphocytes, plasma cells and undifferentiated elements, single granulocytes and normoblasts are found in the fatty bone marrow. With hypoplasia, areas of hematopoietic tissue are found somewhat more often. Characteristic is the uneven distribution of foci of hematopoiesis not only in different parts of the skeleton, but also within the same bone. The structure of the bone tissue is often preserved. Both the early and late phases of the disease are characterized by the deposition of hemosiderin in the spleen, liver (Fig. 1) and bone marrow, less often in the kidneys (Fig. 2), lymph nodes.
Frequent complications of hypoplastic anemia include fibrinous-hemorrhagic pneumonia and necrotic changes in the mucous membranes and serous tissues, skin, and internal organs.
Diagnostics
Examination of the patient is one of the initial moments of diagnosis
Diagnosis begins with specifying symptoms and medical history. A physical examination is performed, during which the doctor examines the mucous membranes, oral cavity, condition of the skin, nails, and hair. A general blood test is prescribed, which will need to be repeated before starting treatment.
- When diagnosing a clinical analysis, the number of blood cells, their characteristics and ratio are taken into account; hemoglobin level.
- A biochemical blood test allows you to determine the values of serum iron, the total iron-binding capacity of serum, and the characteristics of transferrin. Indicators of protein, bilirubin, transaminase, magnesium, sodium, calcium, potassium, urea, and creatinine are also studied.
- A urine test examines the presence of red blood cells, white blood cell levels and protein.
- Diagnostics of a bone marrow sample reveals the number of foci of hematopoiesis and the state of the hematopoietic tissue.
- Additionally, the patient may be referred for an abdominal ultrasound or chest x-ray.
Course and symptoms
The disease can be acute, subacute or chronic. In acute cases, the process begins with violent hemorrhagic diathesis (see Hemorrhagic diathesis), severe general intoxication, and infectious complications. More often there is a gradual increase in symptoms. Adynamia, weakness, dizziness, fatigue, shortness of breath during physical activity appear. stress, sometimes pain in the bones and heart area. There is a waxy pallor of the skin without jaundice, anemia of the visible mucous membranes. Subcutaneous fat tissue is preserved. With the development of deep thrombocytopenia (see), hemorrhages occur on the skin, mucous membranes and fundus of the eye, nasal, gingival, uterine, intestinal, renal and other bleeding. As the process progresses, neurological symptoms may occur due to hemorrhages in the brain. With partial G. a. there is no bleeding. The sizes of the lymph nodes, spleen and liver do not change.
Picture of blood. Anemia is normochromic, normocytic, less often macrocytic in nature. Polychromatophilia is absent, the number of reticulocytes is normal or reduced. Leukopenia can reach 1000 cells in 1 μl or less; it is caused mainly by granulocytopenia and is accompanied by relative lymphocytosis. The absolute number of lymphocytes with severe leukopenia is also reduced. Thrombocytopenia is noted with prolongation of bleeding time (see) and decreased retraction of the blood clot (see Retraction). Plasma blood coagulation factors (see Blood coagulation system) do not change. The serum iron content is increased, the total iron-binding capacity of serum is often increased.
Partial G. a. characterized by hron, normocytic anemia, often without leuko- and thrombocytopenia, with deep reticulocytopenia.
Bone marrow punctate is usually scant; nuclear elements are represented mainly by lymphocytes, a few granulocytes and normoblasts. Megakaryocytes are often absent. Sometimes, despite obvious pancytopenia, the punctate turns out to be quite rich; this is due to the needle entering the site of preserved hematopoiesis. In addition to a purely quantitative deficiency of erythroblastic elements, with G. a. they note signs of their qualitative pathology (dyserythropoiesis), megaloblastoidity, atypical mitoses, multinucleation (see Dyserythropoietic anemia). With partial G. a. bone marrow punctate is rich in nuclear elements, leuko-thrombocytopoiesis is not impaired, but the number of red row cells is often sharply reduced or they are completely absent.
Rice. 3. Microslide of the bone marrow of the ilium (hypoplastic anemia): a sharp predominance of fatty bone marrow over hematopoietic one. The bone marrow cavities are filled predominantly with adipose tissue, myeloid tissue is represented by small black islands; x 280.
Trephine biopsy of the ilium reveals a sharp predominance of fatty bone marrow over hematopoietic bone marrow (Fig. 3), sometimes the absence of the latter.
A study with radioactive iron 59Fe provides the most accurate overall assessment of erythropoiesis: the clearance of the isotope from plasma is slowed down, it accumulates mainly in the liver, and the incorporation of the label into erythrocytes is sharply slowed down.
Symptoms
Depending on the severity of the disturbances in hematopoietic processes, the clinical manifestations and symptoms of aplastic anemia are very diverse: transitional forms of aplastic anemia are distinguished from partial inhibition of the formation of blood cells to severe underdevelopment of the bone marrow.
During the course of the disease, there are 3 main syndromes of aplastic anemia:
- cytopenic;
- septic – necrotic;
- hemorrhagic.
These anemia syndromes can manifest differently in the human body depending on the degree of development of the disease. There are 3 stages of the development of the disease, and the symptoms of aplastic anemia differ in each of them.
Stage I is characterized by the manifestation of nonspecific symptoms that can occur with any other pathological process - chronic fatigue and general weakness. Very often, patients adapt to their existing anemia and turn to a specialist only when the disease begins to progress. This stage is also characterized by such manifestations of hemorrhagic syndrome as uterine, nosebleeds and an increased tendency to bruise (bruises on the body for no reason).
At stage II, patients with aplastic anemia experience pallor of the skin and visible mucous membranes, and sometimes bruising may occur. If the disease becomes acute, in addition to pallor of the skin, hemorrhagic shock, necrosis of the mucous membranes and high fever are also observed. Various inflammatory processes (in particular, pneumonia) are activated in the body.
Usually the liver and spleen do not enlarge, but if an autoimmune form of aplastic anemia is diagnosed, in which the patient’s body produces antibodies to red blood cells, moderate splenomegaly (enlarged spleen) and mild jaundice of the skin and sclera may develop, caused by the presence of hemolytic components in the blood.
Hemorrhagic rashes
Indicators in the blood test
The most pronounced stage of anemia is the third stage, which is characterized by a stormy picture of clinical manifestations. At this stage of development of aplastic anemia, a blood test shows:
- a clinical blood test reveals pronounced anemia (usually normochromic) - the hemoglobin level decreases to 20 - 30 g/l, the concentration of reticulocytes decreases (indicates a decrease in bone marrow functionality);
- leukopenia, granulocytopenia occurs, that is, the level of granular leukocytes in the blood sharply decreases. In this case, the number of lymphocytes does not change;
- decrease in platelets, down to zero;
- Histological examination of bone marrow tissue reveals a catastrophic disappearance of its cells, which are replaced by adipose tissue;
- ESR increases sharply – up to 30 – 50 mm/hour;
- the concentration of iron in the blood serum increases.
Complications
The main complications and direct cause of death of patients are bleeding and infectious and inflammatory processes. Hemorrhages are detected when the number of platelets decreases below a critical level (20,000-30,000 in 1 μl); they can be provoked even by minor injuries (subcutaneous injections, etc.). Infections (pneumonia, etc.) develop with a fairly long-term and pronounced (less than 500 in 1 μl) decrease in the number of neutrophils.
Any fever in patients with G. a., as a rule, is associated with the presence of an infectious focus; Many cases of acute leukemia developing in them have been described. Leukemias against the background of hypoplasia of radiation or benzene origin belong to the group of myeloblastic or myelomonoblastic and are apparently a late result of the leukemogenic effect of the factor that caused anemia. Connection to G. a is much less common. paroxysmal nocturnal hemoglobinuria, although some authors interpret in these cases Hypoplastic anemia as the hypoplastic stage of this form of hemolytic anemia.
Blood tests for aplastic anemia
With aplastic anemia, a blood test can tell a lot, as with any other form of this disease.
The most common type of anemia is iron deficiency, which is why anemia is called iron deficiency. In general, it refers to a disease that provokes iron deficiency or an improper process of its processing within the body. Diagnosis of iron deficiency anemia confirms that it cannot be considered a primary disease. There are additional pathological influences.
Iron deficiency in the body causes improper hematopoiesis. Due to iron deficiency, improper hemoglobin synthesis occurs on the basis of red blood cells.
As a result, their number becomes smaller, as do their functional abilities.
Diagnosis of anemia demonstrates that the iron deficiency variant is the most common in the world. Women most often experience IDA, including due to pregnancy and breastfeeding. In addition, they periodically lose blood, which reduces the amount of iron.
Carrying out analysis
A blood test for anemia is a mandatory test. There are three options available:
- general blood test;
- biochemistry;
- stool occult blood test.
Doctors' recommendations suggest that it is better to undergo all examinations rather than choose just one. Speaking about a general blood test, the indicators determined during it help to understand the condition of all blood cells. The CBC demonstrates the ratio of their volumetric indicator to the liquid blood part. In addition, hemoglobin parameters and the leukocyte formula are determined.
What blood tests are there? 1899
Biochemistry is a laboratory diagnostic that helps to understand the state of systems and internal organs.
Such a study helps to determine the lack of iron in the body, not only pronounced, but also hidden, by determining the level of ferritin and transferrin. The first component demonstrates how much iron reserves remain in the body.
In addition, other indicators are demonstrated, shifts in which may be explained by a lack of iron.
As part of a stool test for occult blood, you can analyze whether there is bleeding in the stomach and intestines. It is important to note that such laboratory diagnostics require special preparation. We are talking about excluding from the diet three days before delivery foods high in iron and medications that are intended to compensate for this deficiency.
It is worth noting right away that most anemia can be corrected, especially if detected at an early stage. In order for tests for anemia to show the correct numbers, it is important to prepare for them correctly. The specifics of preparing for the exam were mentioned above.
If we talk about a blood test for iron deficiency anemia, then everything is somewhat simpler. The day before the test, you should not drink any drink containing alcohol. Blood donation is performed in the morning after a preliminary eight-hour fast.
During this period, it is prohibited to consume any food or drinks other than still water. By agreement with the doctor, any medications other than vital ones are excluded. Drugs containing iron should be excluded three days before the test. Smoking is prohibited half an hour before the test.
It is important to avoid overexertion from a physical and emotional point of view.
What types of anemia are there?
It was already noted above that tests for anemia are different, since there are different types of the disease. In most cases, a diagnosis of iron deficiency anemia is made. There is an iron deficiency in the body.
This may be the result of insufficient intake of it with food, or the result of improper processing of it in the body or prolonged bleeding. The analysis is to determine the reason according to which treatment is selected.
Often this blood picture is observed in pregnant or nursing mothers; at such moments, iron is consumed by the body in large quantities, so it is not difficult to reach an anemic state.
To differentiate this condition, pay attention to hemoglobin, in this case it is shown to be insufficient, the erythrocyte count and the level of iron in the serum are reduced. Treatment of IDA, also called anemia, involves taking iron on a long-term basis.
Hemolytic anemia also occurs. In this case, the disease develops against the background of hemolysis - red blood cell destruction in the blood. Various factors can contribute to this. As an example, poisoning, severe stress, diseases transmitted by inheritance.
For such a diagnosis, a urine test is deciphered, in which hemoglobin is detected. The level of bilirubin in the blood increases. Sometimes red blood cells with altered shapes and other signs are present. Based on the hemogram, the root cause is determined, which needs to be treated.
B12 deficiency anemia, or otherwise pernicious anemia, occurs when there is a deficiency of vitamin B 12. Without this vitamin, the growth and maturation of red blood cells in the bone marrow is impossible. In addition, without it, the proper functioning of the nervous system is impossible.
Pernicious anemia often manifests itself as tingling and numbness present in the fingers, and gait becomes unsteady. In tests, pernicious anemia manifests itself as an increased number of red blood cells with an enlarged shape.
Pernicious anemia is common in older people. You can also find another name for it – megaloblastic anemia. An insufficient amount of B12 is recorded in diseases of the digestive tract, in the presence of parasites such as tapeworms in the body.
All this provokes the diagnosis of megaloblastic anemia.
A little more about the types of anemia
There is also the so-called pernicious anemia, which is called aplastic anemia.
These forms of anemia mean a special group of blood diseases, as a result of which the number of all blood cells decreases, and there are no signs of a tumor process.
This is not a very common disease, but it can affect all genders and ages, but is more common in the older age group. The disadvantage of aplastic anemia is that at least two thirds of those affected do not survive.
Another unusual form is sickle cell anemia. It becomes a consequence of a hereditary disease of the blood system.
We are talking about a defect that operates at the genetic level, as a result of which the formation of normal hemoglobin chains within red blood cells is disrupted.
The hemoglobin that is formed in this case is abnormal; it also has differences in its electrophysiological properties when compared with normal healthy hemoglobin.
As a result of this process, changes in the red blood cells themselves are recorded, which become elongated in length. Among hereditary hemoglobinopathies, SCA is considered the most severe form. The fact is that red blood cells with such a change undergo rapid destruction in the body, often causing severe complications and death.
Hypochromic anemia is a general name for a whole group of diseases in which insufficiency of hemoglobin leads to the formation of a different color indicator of the blood.
Decoding the analysis in this case shows a figure of less than 0.8, and the concentration of hemoglobin in one red blood cell does not reach 330 g/l. Most often, hypochromic anemia develops after IDA. In addition, chronic lead poisoning and a lack of vitamin B6 can lead to such a diagnosis.
You can encounter it after some inflammations of an infectious and non-infectious nature, which lead to disruption of glandular metabolism.
Causes of IDA
If the differential diagnosis of iron deficiency anemia has confirmed the diagnosis, it is important to identify the cause of the pathology. It was already noted above that IDA can be provoked both by a lack of iron in food and by its improper processing by the body.
In addition, one cannot rule out increased iron needs of the body, congenital iron deficiency, or impaired absorption or transferrin synthesis. Massive blood loss, alcoholism, and taking a number of medications have a negative impact.
If we talk about the lack of iron supplied with food, then this problem most often occurs during prolonged fasting, among vegetarians, and with monotonous diets that do not involve a large amount of animal products.
An increased need for iron is a normal sign when it comes to pregnant or nursing mothers. During such periods, the amount of it in food should increase. Moreover, if we are talking about multiple pregnancies, then the figures for iron consumption should increase several times more.
Children suffering from congenital iron deficiency are born to mothers with severe iron deficiency anemia, multiple pregnancies or prematurity. In this case, the child encounters symptoms of IDA already in the first weeks of life.
When we talk about improper absorption of iron, we mean the work of the duodenum, namely problems with the mucous membrane of this intestinal section. A variety of gastrointestinal diseases cause damaged mucous membranes, which affects the rate at which iron is absorbed.
This includes a number of hereditary diseases, including celiac disease, which implies gluten intolerance. This may be inflammation of the mucous membrane that covers the small intestine, infectious problems, atrophic gastritis, as a result of which the size of the mucous membrane is reduced, which reduces functionality.
Gastritis of the autoimmune type cannot be ruled out. The disease is provoked by impaired immune function and antibody production.
At the same time, they attack the body’s own cells, in particular, the cells that cover the gastric mucosa, against which they are destroyed.
This diagnosis can also be made to people who have had their stomach or part of the small intestine removed. Crohn's disease, cystic fibrosis and cancer problems cannot be ruled out.
When it comes to improper synthesis of transferrin, in most cases such problems are provoked by various kinds of hereditary diseases.
Transferrin is responsible for the transfer of iron to various organs; as a result, the red bone marrow also suffers, since red blood cells cannot be synthesized.
Liver cells are responsible for the synthesis of transferrin, so their various lesions can cause a reduced amount of transferrin, which will provoke the symptoms of IDA.
Increased blood loss can also lead to iron deficiency. In this case, it is most often chronic small blood losses that are meant.
The fact is that one-time, even massive blood losses do not cause such a problem, since iron is present in the body in the form of reserves, which are intended to compensate for such losses.
When we are talking about chronic bleeding inside, which is not immediately noticeable and lasts a long time, a person can lose a milligram of iron every day and so on for a long time.
Chronic blood loss can develop against the background of gastric or duodenal ulcers, hemorrhoids, ulcerative colitis, Crohn's disease, and intestinal polyposis.
Gastrointestinal tumors, endometriosis, and systemic lupus erythematosus have the same effect. Overly active donors who donate blood more than four times a year may also encounter this diagnosis.
It is important to detect the immediate cause of blood loss, otherwise IDA is inevitable for the patient.
Alcoholism is also not iron's best friend. Long-term and frequent consumption of alcoholic beverages damages the gastric mucosa. In addition, ethyl alcohol inhibits the hematopoietic processes that take place in the red bone marrow, which increases anemic manifestations.
Taking certain medications cannot be ruled out as a cause of IDA. The fact is that for some medications, disruption of the processes of absorption and glandular utilization is a side effect. Most often, this problem can be encountered during a long course of treatment with large doses of the drug.
What does a general blood test show during pregnancy? 34404
Popular medications such as non-steroidal anti-inflammatory drugs can affect iron levels. The fact is that such drugs seem to thin the blood, which is fraught with the formation of chronic internal bleeding. In addition, their uncontrolled use is dangerous for stomach ulcers.
The group of drugs that are dangerous, from this point of view, also includes antacids, which reduce or neutralize the rate of gastric juice secretion. It contains hydrochloric acid, which is required for normal absorption of iron.
Iron-binding drugs help bind and remove iron from the body. In this case, we are talking not only about free iron, but also about the body’s reserves.
When talking about the symptoms of anemia associated with iron deficiency, doctors most often draw attention to pale skin and lips, weakness, and fatigue.
Due to the lack of oxygen in the blood, the pulse increases, which exceeds 90 beats per minute. Signs may vary depending on the type of anemia you have.
Source: https://1diagnos.ru/laboratornye-issledovaniya/aplasticheskaya-anemiya.html
Diagnosis
The diagnosis is based on the presence of pancytopenia and bone marrow punctate or trephine, which is poor in nuclear cells, in the absence of enlarged lymph nodes, spleen and liver. G. a. should be differentiated ch. arr. with pancytopenia of other origin. The detection of young white or red cells in the blood, even a slight enlargement of the spleen should always raise doubts about the diagnosis of G. a. In these cases, one can assume the presence of aleukemic forms of leukemia (see), cancer metastases to the bone marrow, myeloma (see). The diagnosis is usually made on the basis of bone marrow aspirate examination; multiple myeloma, in addition, is characterized by characteristic changes in serum and (or) urine proteins. Pancytopenia in myelofibrosis (see Osteomyelofibrosis) is accompanied, in contrast to G. a., by enlargement and myeloid metaplasia of the spleen. Addison-Biermer's anemia (see Pernicious anemia) is distinguished from G. a. the presence of glossitis, neurol, disorders, achlorhydria, severe bone marrow megaloblastosis, good therapeutic effect from vitamin B12. The diagnosis of paroxysmal nocturnal hemoglobinuria, even in its early, hypoplastic stage, is confirmed by positive results of sucrose and acid tests (see Hemolytic anemia). In the subacute variant of lymphogranulomatosis (see), pancytopenia is accompanied by fever, there is no severe lymphocytosis, and sometimes Berezovsky-Sternberg cells can be detected in bone marrow trephine. Pancytopenia due to hypersplenism is usually accompanied by an enlarged spleen.
Treatment of hypoplastic anemia
Treatment tactics depend on the cause of anemia
- First of all, the cause of the disease is eliminated (cessation of taking medications, interactions with toxic substances).
- For autoimmune forms, glucocorticoid drugs are prescribed. In the severe stage, the best result is achieved by removing the spleen. There is also a new method that has proven its effectiveness - complete replacement of immune cells.
- In the presence of viral infections that cause hypoplastic anemia, treatment may consist of taking antiviral drugs, antibiotics (if bacterial infection is present), and glucocorticosteroids (for complications of various types).
- In case of intoxication from interaction with toxic substances, medications are prescribed that stop the effect of the poison on the body - antidotes.
- The general purpose in the treatment of hypoplastic anemia is transfusion. If certain blood cell counts decrease, only red blood cells, platelets, and leukocytes can be transfused. The exception is autoimmune anemia.
- Taking medications that restore hematopoiesis. For example, cyanocobalamin is a drug that promotes the maturation of red blood cells and the activation of platelets.
- Bone marrow transplantation is the only effective way to resuscitate bone marrow function in extremely severe forms of the disease.
Treatment
There are no radical cure methods for hypoplastic anemia, but therapeutic measures help to lengthen the life expectancy of patients. If etiol is identified, the factor must be stopped from further exposure. To combat anemia, transfusions of blood or red blood cells are indicated; this measure should not pursue the goal of complete normalization of red blood parameters - it is sufficient to maintain them at a level compatible with cardiovascular compensation. The number of transfusions should be minimal to reduce the risk of transfusion siderosis (when 450 ml of blood is transfused, 200-250 mg of iron is introduced into the body), hepatitis and other complications. To avoid isosensitization by leukocyte and platelet antigens, it is advisable to transfuse washed red blood cells. With multiple blood transfusions, the development of isosensitization by erythrocyte antigens is common; in these cases, blood for transfusion is selected using an indirect Coombs test (see Coombs reaction).
In the case of a combination of anemia with hemorrhagic diathesis, transfusions of freshly citrated blood or direct blood transfusions in a single dose of at least 500 ml are indicated. A more pronounced hemostatic effect is achieved by platelet concentrates obtained using a blood cell separator from one donor or by centrifugation of a large number of blood units taken from different donors. The hemostatic effect of platelet concentrates manifests itself if the patient’s platelet count can be increased to at least 15,000–20,000 in 1 μl of blood. With multiple transfusions of platelet concentrates, especially those obtained from the blood of different donors, isosensitization to platelet antigens inevitably develops and a decrease in the therapeutic effectiveness of transfusions is noted. Therefore, it is advisable to use a limited number of individuals as donors, preferably relatives, who are maximally compatible with the antigens of the HL-A system (see Blood groups, leukocyte antigens).
Among medications, only anabolic steroids have the property of stimulating erythropoiesis (see). A necessary condition for achieving a therapeutic effect is considered to be the duration of use of hormones in a sufficiently high dose (for example, methyltestosterone or Nerobol 1-2 mg per 1 kg of body weight per day orally for 3-4 months) [McCready (K.V. McCredie), 1969]. The first manifestation to treat. the effectiveness of the drugs may be an improvement in peripheral blood parameters. If signs of side effects appear (fluid retention, liver damage), hormones are canceled. For the treatment of G. a. corticosteroids are also used (prednisolone 0.5-1.0 mg per 1 kg of body weight or equivalent doses of other steroids) Ch. arr. for the purpose of hemostatic action (reducing vascular permeability); For this purpose, others also use the so-called. vascular strengthening agents - ascorbic acid, rutin, calcium preparations. In order to reduce hemosiderosis of organs and tissues, drugs such as desferal can be prescribed.
Numerous attempts to transplant donor bone marrow into patients, undertaken without proper immunological selection (only for the main erythrocyte antigens), were unsuccessful, that is, they ended in graft rejection due to biol incompatibility. Cases of genuine transplantation are few. ED Thomas et al. (1974) carried out in four patients with G. a. successful transplantation of syngeneic bone marrow obtained from identical twins of patients, which led to complete recovery; the operation was performed without special preparation due to the antigenic identity of recipients and donors. However, such a possibility is casuistic. Allogeneic transplants (from donors compatible according to the Hl-A system) require complex preparation of recipients to prevent graft rejection (general irradiation or administration of cyclophosphamide for the purpose of immunosuppression), as well as postoperative use of cytostatics to suppress immunol, graft-versus-host disease (see. Immunological incompatibility). Of the 24 patients with G. a., described by Thomas et al., 12 lived for St. 3 months; their engraftment of the transplant was proven as a result of sex chromatin analysis (see). In patients who have previously received numerous blood transfusions, the chances of graft engraftment are reduced due to the formation of isoantibodies. The complexity of the bone marrow transplant technique makes it accessible only to certain specialized institutions.
The question of the effectiveness of splenectomy has not been finally resolved. In case of severe hemorrhages, surgery is contraindicated due to high mortality. Probably, splenectomy (see) is more appropriate in patients with increased sequestration of platelets and red blood cells in the spleen, proven by radioisotope method, and with preservation of foci of normal hematopoiesis in the bone marrow.
Folate deficiency anemia
A general examination reveals a decreased state of the red blood cell. The diameter of this microelement will be more than 12 microns. Folate deficiency is also characterized by reticulopenia, a decrease in platelet and leukocyte counts. In addition to these manifestations, folate deficiency pathology causes lymphocytosis. ESR increases its intensity. Folate deficiency pathology is included in the megaloblastic subgroup. Due to the low percentage of folic acid, the division of mature blood cells stops, and folate deficiency develops.
The hyperchromic group of the disease is characterized by a reduced number of red blood cells. At the same time, the hemoglobin level practically does not change. Consequently, red blood cells experience an excess of this substance. Therefore, it causes the red blood cells to become intensely red. Diagnosed hypochromic is provoked by a reduced level of hemoglobin in red blood cells. Diagnosis of the disease comes in laboratory conditions. Deciphering the blood test helps determine whether the patient has it. To do this, it is necessary to determine the ratio of red blood cells, hemoglobin and color.
Author: E. Kubina
Prevention
Persons professionally associated with exposure to myelotoxic factors (sources of ionizing radiation, benzene production) should be under constant medical supervision. Carrying out cytostatic treatment for tumors and other diseases requires regular monitoring of blood composition and timely termination if there is a threat of hematopoietic hypoplasia. The use of potentially dangerous drugs, primarily chloramphenicol (see), should be limited to direct indications and be monitored by blood composition. Prevention of hemorrhages with already developed hemorrhage. includes hormonal suppression of the menstrual cycle in women with massive menorrhagia, replacement (if possible) of injection medications with oral ones, sparing the mucous membranes (excluding rough foods, replacing hard toothbrushes with cotton swabs). To prevent infectious complications in deep granulocytopenia (see Leukopenia), it is recommended to suppress intestinal autoflora with non-absorbable antibiotics; patients should be kept in aseptic or similar conditions, and personal hygiene should be monitored.
Mechanism of anemia development
Anemia is a syndrome characterized by a sharp decrease in the amount of hemoglobin in the blood. As you know, hemoglobin carries oxygen from the lungs to the tissues. Hemoglobin, in turn, is part of red blood cells - red blood cells synthesized in the bone marrow from hematopoietic stem cells.
In aplastic anemia, the bone marrow stops producing new red blood cells or dramatically reduces their production. This is due to a decrease in the number of hematopoietic stem cells or a violation of their functionality.
Typically, the production of not only red blood cells, but also immune cells - leukocytes, as well as platelets, which are responsible for blood clotting, decreases. A condition called panhemocytopenia occurs. Thus, in aplastic anemia, the production of all major blood cells stops or is seriously reduced, which poses a serious threat to life. After all, red blood cells live in the blood for only three months, platelets - 1-2 weeks, and white blood cells - no more than a day.
Main blood test indicators and their values indicating anemia
At the initial stage, to determine the presence of any anemia, the main indicators of the blood test are compared with reference values. The main values of the studied quantities are presented in the table:
| Patients | Hemoglobin, g/l (HB) | Red blood cells, million/µl (RBC) | Color index | Reticulocytes, % (RTC) |
| Men | 130 – 160 | 3,8 – 5,6 | 0,83 – 1,05 | 5,1 – 18,0 |
| Women | 120 – 140 | 3,7 – 5,3 | 5,0 – 17,0 | |
| Teenagers (14-18 years old) | 125 – 145 | 3,7 – 5,2 | 0,9 – 1,0 | 4,8 – 18,0 |
| Children (10-14) | 120 – 140 | 3,8 – 5,0 | 4,8 – 18,0 | |
| Children (5-10) | 110 – 135 | 3,9 – 5,1 | 4,8 – 18,0 |
Hemoglobin level
This is the main coloring substance that is part of red blood cells, which is responsible for the transfer of oxygen. A reduced hemoglobin component indicates the presence of anemia of various etiologies.
Based on the indicators of quantitative deviation of hemoglobin from the reference values, the nature of anemia is identified according to the degree of intensity:
- light – hemoglobin content from 110-90 g/l;
- average – from 90-70 g/l;
- heavy – less than 70 g/l.
Other indicators also help determine the nature and possible causes of anemia.
Red blood cells
Red anucleate blood cells that are disc-shaped. Due to their biconvex shape, red blood cells can deform to accommodate narrow capillaries. Red blood cells deliver oxygen from the lungs to all tissues and take away carbon dioxide. Low levels of these cells characterize any type of anemia.
Reticulocytes
These cells are an immature form of red blood cells. They are detected in the bone marrow and are found in some quantities in the peripheral blood. An increase in the proportion of reticulocytes indicates the destruction of red blood cells, which indicates the progression of anemia. The calculation is carried out as a percentage of all red blood cells. The value of reticulocytes helps to assess the severity of the disease.
Color index
This indicator is needed to determine the degree of hemoglobin saturation of blood cells (erythrocytes). If it is below normal, this may indicate the presence of anemia. Based on the CPU value, they are distinguished:
- hypochromic anemia (less than 0.8);
- normochromic anemia (0.8-1.05);
- hyperchromic anemia (over 1.05).
This data helps to identify the type of disease. A high color index indicates folate deficiency and B12 anemia. A normal CP value occurs in acute posthemorrhagic anemia. A reduced rate indicates iron deficiency.
For a more accurate diagnosis of types of anemia, other nonspecific blood test indicators are determined.
Red blood cell indices
MCV
– average volume of erythrocyte. The identified values indicate the presence of such anemia:
| Type | MCV value | Type of anemia |
| Normocytic | from 80-100 fl | Hemolytic |
| Microcytic | less than 80 fl | Iron deficiency |
| Macrocytic | more than 100 fl | Folievo and B12 are deficient |