What is this analysis?
If you explain the features of arterial blood gas analysis in understandable language, then it can be noted that this test shows how well the lungs transport oxygen to the blood.
As blood passes through the lungs, it becomes oxygenated. The blood then transports oxygen throughout the body. In parallel with this, carbon dioxide is removed from the blood with the help of the lungs. Blood sampling is performed precisely from the artery for the reason that arterial blood does not yet have time to transfer oxygen to other tissues, as a result it becomes possible to really assess the ratio of these gases in the body.
Thus, thanks to a blood gas test, you can find out about its acidity, the degree of oxygen and carbon dioxide content. Based on the data obtained, we can draw a conclusion about the work of the lungs, namely, how they deliver oxygen to the body.
Blood passing through the lungs is saturated with oxygen. Then it spreads throughout the body to all organs. At the same time, the blood is cleared of carbon dioxide by the lungs. It is important to take the test before the blood has cleared, so it is taken from the artery. This allows you to measure the actual concentration of gas impurities in the blood.
Arterial blood gases: metabolic, respiratory acidosis, alkalosis
- There are four main types of acid-base imbalance: Respiratory acidosis
- Metabolic acidosis
- Respiratory alkalosis
- Metabolic alkalosis
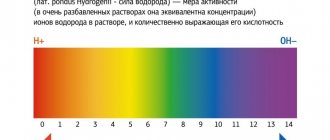
Blood pH control
Normal blood pH is somewhat alkaline (7.35-7.45). In order to function normally, the body maintains the blood pH at approximately 7.4. There are three mechanisms by which the body maintains acid-base balance within this narrow range:
- Intracellular and extracellular buffer systems
- Renal regulation
- Regulation with the help of the lungs.
The most important pH buffer systems include hemoglobin, carbonic acid (a weak acid that is formed when CO2 dissolves) and bicarbonate (its weak base). The bicarbonate buffer is effective because the concentrations of its components can be independently adjusted. Its key components are CO2 and HCO3—.
- The lungs regulate the partial pressure of CO2 in the blood (pCO2) by regulating alveolar ventilation
- The kidneys regulate HCO3 concentrations by regulating renal carbonic acid excretion and bicarbonate reabsorption
Henderson-Hasselbach EquationBlood gas analyzers directly measure pH and pCO2 levels. HCO3 level—calculated using the Henderson-Hasselbach equation. This equation shows that the pH value is determined by the ratio of the concentration of HCO3- to pCO2, and not just the value of one of the components.
The simplified version of the equation below shows the relationship between these three quantities. If you remember this option, it will help you understand compensatory changes, which are described later in the module.
Definitions
Acidemia
Occurs when the blood pH is below 7.35.
Alkalemia
Occurs when the blood pH is above 7.45.
Acidosis
- This is the process that causes acids to build up in the blood
- Does not necessarily lead to abnormal pH levels
- From the Henderson-Hasselbach equation, you can see that acidosis can be caused by a decrease in HCO3—or an increase in pCO2:
- Occurring in isolation it leads to acidemia
- When developing simultaneously with alkalosis, the resulting blood pH may be normal, increased or decreased
Alkalosis
- This is a process that causes alkali to accumulate in the blood
- Does not necessarily lead to abnormal pH levels
- From the Henderson-Hasselbach equation you can see that alkalosis can be caused by an increase in HCO3—or a decrease in pCO2:
- Occurring in isolation it leads to alkalemia
- When developing simultaneously with acidosis, the resulting blood pH may be normal, increased or decreased
Base excess
Base excess is the amount of base or acid required to titrate one liter of blood to a pH of 7.4 at a constant pCO2 of 5.3 kPa. In the context of acidosis, a negative base excess value indicates the presence of a metabolic component.
Why and how to measure ABG
Why measure arterial blood gas composition?
You should determine your blood gas composition in order to:
- Determine acid-base balance
- Determine oxygenation level (arterial pO2 provides information about the efficiency of gas exchange and is more accurate than determining peripheral oxygen saturation)
- Diagnose and establish the severity of respiratory failure (pCO2 level provides information regarding ventilation of the lungs)
- Decide on treatment, such as oxygen or noninvasive ventilation for patients with chronic obstructive pulmonary disease (COPD) or therapy for patients with diabetic ketoacidosis.
The four main acid-base disorders are:
- Respiratory acidosis
- Metabolic acidosis
- Respiratory alkalosis
- Metabolic alkalosis.
Interpretation of ABG results
A step-by-step approach to interpreting arterial blood gas results
The following approach is a systematic way to help you correctly interpret arterial blood gas results (Table 1).
Table 1. Five steps for interpreting arterial blood gas results
| Step 1 | Is acidemia or alkalemia present? |
| Step 2 | Is the primary disorder respiratory or metabolic? |
| Step 3 | In cases of metabolic acidosis, is an increased anion gap present? |
| Step 4 | Is there compensation available? If so, is it appropriate? |
| Step 5 | What is the alveolar-arterial gradient? Look at arterial pO2 in the context of inspired oxygen concentration and arterial pCO2 |
First of all, you need to know the normal values (Table 2). Please note that these rates vary slightly between hospitals, so always use your hospital's normal rates.
Table 2: Normal arterial blood gas values
| Arterial pCO2 | 4.5-6.0 kPa |
| Arterial pO2 | 11.0-13.0 kPa |
| HCO3— | 22.0-28.0 mmol/l |
| Base excess | -2.0 to +2.0 |
| Anion gap | 8.0-16.0 mmol/l |
| Chlorides | 98.0-107.0 mmol/l |
Step 1: Acidemia or Alkalemia?
Look at the pH. If he:
- below 7.35, then the patient has acidemia
- above 7.45 - the patient has alkalemia.
If the pH is normal, then look at the pCO2 level and the HCO3- concentration. If one or two indicators deviate from the norm, then the patient may have a mixed disorder.
Step 2: Respiratory or Metabolic?
Is the primary disorder respiratory or metabolic?
Look at pH, pCO2 and HCO3- concentration.
- If the pH is below 7.35 then acidosis causes acidemia and: if the pCO2 level is elevated then it is primary respiratory acidosis
- if the concentration of HCO3- is reduced, then this is primary metabolic acidosis.
- if the pCO2 level is reduced, then this is primary respiratory alkalosis
Step 3: Causes of Acidosis
In the case of metabolic acidosis, is there an increased anion gap?
Determining the type of acidosis will help you narrow down possible underlying causes.
What is an anion gap?
In the body, the number of cations and anions is the same. Blood tests measure most cations and only a few anions. Therefore, when all the measured anions and cations are added, an interval remains that represents unmeasured anions such as plasma protein, albumin.
Since Na+ is the main cation measured and Cl- and HCO3- are the main anions measured, the anion gap is calculated using the following formula:
Na+ — (HCO3— +Cl—)
Normally, the anion gap is 8-16 mmol/l.
Some hospital laboratories include K+ when calculating the anion gap. If K+ is included, the normal range is 12-20 mmol/L.
The main causes of acidosis with a high anion gap (more than 16 mmol/l) are given in Table 3.
Table 3. Main causes of acidosis with a high anion gap (more than 16 mmol/l)
| Increased production of endogenous acids |
|
| Increase in the amount of exogenous acids |
|
| Inability to excrete acids |
|
The main causes of acidosis with a normal anion gap (8-16 mmol/l), as a rule, are associated with an increase in plasma Cl- levels and are presented in table 4.
Table 4. main causes of acidosis with normal anion gap (8-16 mmol/l)
| Loss of bicarbonate |
|
| Impaired renal acid excretion |
|
How to correct the anion gap in patients with low albumin concentration?
Of the 8-16 mmol/L anion gap, as a rule, 11 mmol/L is due to albumin. Decreasing albumin concentration may reduce the initial anion gap. A patient with a low albumin concentration may have a normal anion gap in the presence of pathology that is usually accompanied by an increased anion gap.
The anion gap decreases by approximately 2.5 mmol/L for every 10 g/L decrease in albumin levels.
Step 4: Is there compensation?
Compensation means the body's reaction aimed at restoring normal acid-base balance. Standard compensation measures are:
- Buffers that include hemoglobin, plasma proteins, bicarbonate and phosphate. This reaction occurs within a few minutes.
- A respiratory response that develops over a period of minutes to several hours
- A kidney reaction that may develop over several days.
Why is recognizing compensation important?
Recognizing compensation will help you differentiate between the primary disorder and blood gas abnormalities that arise only from the primary disorder. For example, a hyperventilating patient who reduces pCO2 levels solely to compensate for metabolic acidosis likely has partially compensated metabolic acidosis rather than primary respiratory alkalosis.
Although patients with isolated mild disorders and fully compensated patients may have a pH in the normal range (7.35-7.45), a normal pH and abnormal HCO3- and pCO2 should lead you to suspect a mixed acid-base disorder.
It may be difficult for you to decide whether acid-base abnormalities are caused by a mixed disorder or just compensation. It is advisable to be aware of the possible degree of compensation for the primary disorder. If the change in one parameter is beyond the expected changes, then there is likely a mixed disorder (see Table 5). Compensatory reactions in metabolic disorders are not as predictable as in respiratory disorders.
Table 5. Summary: Compensatory Responses
| Acid-base imbalance | Primary chemical change | Compensatory reaction | Amount of compensation |
| Respiratory acidosis | ↑ pCO2 | ↑ HCO3— | For every 1.3 kPa increase in pCO2 above 5.3 kPa in acute respiratory acidosis:
For every 1.3 kPa increase in pCO2 above 5.3 in chronic respiratory acidosis:
|
| Respiratory alkalosis | ↓pCO2 | ↓HCO3— | For every 1.3 kPa decrease in pCO2 level below 5.3 kPa in acute respiratory alkalosis:
For every 1.3 kPa decrease in pCO2 level below 5.3 kPa in chronic respiratory alkalosis:
|
| Metabolic acidosis | ↓HCO3— | ↓pCO2 | |
| Metabolic alkalosis | ↑ HCO3— | ↑ pCO2 |
Compensation always occurs in the same direction as the primary chemical change. This is because compensatory reactions are based on maintaining the ratio of HCO3- to pCO2 concentration. Remember the Henderson-Hasselbach relationship: pH ~ HCO3-/pCO2.
In chronic disorders, the amount of compensation is greater, with further more effective pH maintenance. Knowing about possible changes in the metabolic compensation of primary respiratory disorders, it will be easier for you to diagnose mixed acid-base imbalances.
Metabolic compensation
Metabolic compensation takes several days. It occurs in two stages:
- Cellular buffering that occurs within minutes or hours. This only slightly increases plasma bicarbonate (HCO3—) levels.
- Renal compensation, which occurs within three to five days.
As a result, different types of responses are observed in acute and chronic disorders.
- In respiratory acidosis, renal excretion of carbonic acid and reabsorption of bicarbonate are increased.
- Respiratory alkalosis of the kidney is compensated by reducing bicarbonate reabsorption and ammonium excretion
Respiratory compensation
Respiratory compensation lasts for hours. Maximum respiratory compensation for metabolic disorders lasts from 12 to 24 hours. This reaction begins within the first hour and ends within 12 to 24 hours.
- In metabolic acidosis, stimulation of central and peripheral chemoreceptors that control breathing leads to an increase in alveolar ventilation. This, in turn, causes compensatory respiratory alkalosis
- In metabolic alkalosis, it is difficult to provide hypoventilation for the purpose of compensation. With hypoventilation, oxygenation is also impaired. Therefore, the respiratory system rarely maintains pCO2 above 7.5 kPa. A large value indicates a mixed disorder: it is more likely a metabolic alkalosis with respiratory acidosis, rather than a compensated metabolic alkalosis
Mixed acid-base balance disorders
Mixed acid-base imbalance occurs when more than one primary acid-base imbalance is present at the same time. They often occur in patients in hospital. With a good knowledge of compensatory mechanisms and the degree of compensation, you will be able to identify these disorders. Please note. that it is impossible to have both respiratory alkalosis and respiratory acidosis.
You should suspect mixed acid-base imbalance when:
- A compensatory reaction develops, but the level of compensation is insufficient or too pronounced
- pCO2 and HCO3- concentration deviate from the norm in opposite directions (one increases while the other decreases). In simple acid-base imbalances, the direction of the compensatory response always coincides with the primary chemical pathological change
- The pH is normal, but the pCO2 or HCO3 concentration is abnormal. In simple acid-base imbalances, the compensatory response rarely returns the pH to normal levels. In this case, a mixed disorder should be suspected
As a general rule:
- When pCO2 is elevated and HCO3- concentration is decreased, respiratory acidosis and metabolic alkalosis occur simultaneously.
- If pCO2 is reduced and the concentration of HCO3 is increased, then there is simultaneously respiratory and metabolic alkalosis.
Step 5: Ahh Gradient
What is the alveolar-arterial gradient?
A-and the gradient is the difference between the calculated alveolar pO2 and the measured arterial pO2. Arterial pO2 is a function of gas exchange and fractional inspired air O2 concentration (FiO2). Therefore, the normal range varies.
Calculation of Aa allows you to determine whether the measured arterial oxygen value is normal for the patient:
- Height
- Percentage of inspired oxygen
- Breathing rate.
This provides bedside assessment of gas exchange.
This allows you to calculate the efficiency of oxygen getting from the alveoli into the arterial bloodstream. Alveolar pO2 is always higher than arterial pO2. In healthy people, the gradient is 2-4 kPa. An increased gradient indicates a violation of gas exchange, since values above 4 kPa are pathological.
Getting Aa gradient
When breathing air at sea level, the partial pressure of inhaled oxygen is 21 kPa. It decreases to 20 kPa when saturated with water vapor from the upper respiratory tract (PiO2). In the alveoli, O2 is absorbed and replaced by CO2, which further reduces alveolar pO2 to ~13-14 kPa.
The ratio of pCO2 produced to pO2 consumed is determined by the respiratory coefficient. Its value is 0.8. Therefore, alveolar pO2 is calculated by subtracting alveolar pCO2 from PiO2. The pCO2 value increases slightly due to the respiratory coefficient.
Alveolar pO2 = inspiratory pO2 - alveolar pCO2 / 0.8 = inspiratory pO2 - alveolar pCO2 x 1.2
Since alveolar pCO2 is approximately equal to arterial pCO2, then:
Alveolar pO2 = inspiratory pO2 - arterial pCO2 x 1.2.
Since the Aa gradient is the difference between the calculated alveolar pO2 and the measured arterial pO2, you can calculate the gradient in kPa by subtracting the arterial pO2 from the calculated alveolar pO2:
- Alveolar pO2 = PiO2 - arterial pCO2 x 1.2
- Aa gradient = alveolar pO2 - arterial pO2
- PiO2 = effective inspiratory pO2.
Common acid-base disorders
Respiratory acidosis
It is a clinical disorder caused by alveolar hypoventilation (i.e., respiratory failure). Ineffective ventilation quickly leads to elevated arterial pCO2. The main reasons are presented in Table 6.
Table 6. Main causes of respiratory acidosis
| Central depression of the activity of the respiratory center |
|
| Neuromuscular disorders that cause weakness of the respiratory muscles |
|
| Pathologies of the chest wall or chest |
|
| Diseases that affect gas exchange |
|
| Airway obstruction |
|
Respiratory alkalosis
It is a clinical disorder that is caused by alveolar hyperventilation. Respiratory alkalosis can be acute or chronic. The main reasons are presented in Table 7.
Table 7. Main causes of respiratory alkalosis
| Central nervous system stimulation |
|
| Hypoxemia or tissue hypoxia |
|
| Lung disease |
|
| Medicines (respiratory stimulants) |
|
Metabolic acidosis
It is a clinical disorder characterized by a relative increase in the total acid content in the body. You should consider conditions that may be a sign of an underlying disease that is affecting the body. Determining this underlying disease is key to initiating appropriate therapy.
There are two types of metabolic acidosis:
- With high anion gap
- With normal anion gap.
Metabolic alkalosis
It is a relatively common clinical problem. It is characterized by high levels of bicarbonate. The main reasons are presented in Table 8.
Table 8. Main causes of metabolic alkalosis
| Loss of hydrogen ions |
|
| Intracellular redistribution of hydrogen |
|
| Excretory alkalosis |
Note:
|
Measurable Indicators
Taking a blood gas test allows you to study the following indicators:
- Partial pressure of oxygen. This value is responsible for how easily oxygen is transported from the lung tissues to the blood.
- Partial pressure of carbon dioxide. Allows you to study how easily carbon dioxide is removed from the blood.
- Acidity. The acidity level shows the amount of hydrogen ions in the blood.
- Bicarbonate. This is a substance that helps maintain the required level of acidity in the blood.
The importance of blood gas parameters for the body
The gas composition of the blood is one of the indicators of homeostasis (constancy) of the body.
Of course, it’s not worth checking the level of gases in your blood for everyone. There are special indications for this. Almost always, blood gases are determined in a hospital setting. Usually, in urgent (emergency or advanced) conditions, such an analysis becomes necessary. The blood gas composition helps the doctor understand the patient’s prognosis and give a correct assessment of the effectiveness of the therapy.
Indicators of blood gas homeostasis and their interpretation
Indicative is not only and not so much the volumetric percentage of carbon dioxide or oxygen, but the partial pressure and, ultimately, the percentage of blood saturation with oxygen calculated according to the formula.
Doctors are interested in 6 indicators:
- percentage of oxygen (norm - 10.5-14.5 volume %);
- percentage of carbon dioxide (normal value - 44.5 - 52.5 volume%);
- partial pressure of oxygen - pO2 (35-46 mm Hg);
- partial pressure of carbon dioxide - pCO2 (normal limits - 81-99 mm Hg);
- oxygen capacity of hemoglobin (about 20% by volume);
- % oxygen saturation (usually 61-70%).
Transport of blood gases throughout the body
The partial pressure of gases is the pressure at which physical dissolution of the gas in the blood begins. This means that oxygen at this pressure works effectively in the body. If pO2 deviates upward or downward, this indicates the presence of factors or diseases that prevent tissues from using oxygen for its intended purpose.
Important. Since current diagnostic capabilities make it possible to determine only the gas composition of venous blood, the standards of resuscitation and surgery are focused on it.
Blood gas analysis is performed using a special device - van Slyke. The blood is collected into a special tube or syringe, the inner surface of which is treated with heparin or potassium oxalate to prevent clotting.
Cartridge blood gas analyzer
100% oxygen saturation during resuscitation is not always good. After all, carbon dioxide contained in the blood activates the respiratory center, which means it regulates the frequency and depth of breathing.
Important. Carbon dioxide stimulates chemoreceptors in the carotid sinus of the carotid artery. This allows you to maintain blood pressure at the proper level and the functioning of the respiratory center.
Carotid sinus at the site of bifurcation (bifurcation) of the carotid artery
The relationship between the content of blood gases and pathological diseases (conditions) of the body
There is a direct correlation between the gas composition of the blood and disturbances in the cardiopulmonary system, which are caused by various diseases.
Determination of blood gas composition is necessary for diagnosis:
- hyperventilation (primary and artificial - from a ventilator);
- respiratory failure.
Primary hyperventilation is most often associated with mental characteristics and excitability of the autonomic nervous system. Panic attacks and unmotivated fear can begin with a feeling of difficulty breathing and lack of air, resulting in convulsive breaths, coughing and sniffling. Hyperventilation is also accompanied by heart pain and muscle stiffness.
Important. However, hyperventilation can be a consequence of thyroid disease, congenital connective tissue dysplasia, and even heart problems. In any case, a clear differential diagnosis is needed.
The relationship between stress and hyperventilation syndrome
Diseases for which blood gas parameters are diagnostically decisive:
- obstructive pulmonary diseases (chronic bronchitis, asthma, occupational lung diseases - asbestosis, silicosis, silicosis, etc.);
- prolonged forced stay on artificial ventilation;
- septic conditions (infectious complications);
- arteriovenous aneurysms and malformations (congenital and traumatic), in which mixing of venous and arterial blood occurs.
Important. Changes in the gas composition of the blood at the sites of traumatic aneurysms greatly help vascular surgeons determine the degree of openness of the arteriovenous anastomosis and, accordingly, the degree of mixing of arterial blood with venous blood. For example, the amount of O2 in a vein near a pathological anastomosis can reach 18 and even 20 vol.%, the percentage of saturation can reach 80, or even 93.
To assess the traumatic injury and the effectiveness of the surgical intervention, blood is taken from a healthy (control) section of the vein and from a section of the vein close to the pathological arteriovenous shunt.
Arteriovenous pathological anastomosis with mixing of blood
Usually, in urgent (emergency or advanced) conditions, such an analysis becomes necessary. The blood gas composition helps the doctor understand the patient’s prognosis and give a correct assessment of the effectiveness of the therapy.
We recommend studying similar materials:
- 1. Hemostasis system: why take a blood clotting test
- 2. How to choose a diet based on your blood type: losing weight together
- 3. Reasons for an increase or decrease in neutrophils in a blood test in children?
- 4. Norms for the content of neutrophils in the blood and what functions they perform
- 5. What do elevated eosinophils mean in a blood test in adults?
- 6. Proper nutrition for high levels of bilirubin in the blood
- 7. Low level of total bilirubin in the blood: reasons for the decrease
Source: //MoyaKrov.ru/sostav/pokazateli-gazovogo-sostava-krovi/
Features of the analysis
An analysis of arterial blood gases does not require special preparation; the only thing that needs to be done is to inform the doctor about the medications used and the presence of various types of allergic reactions.
Before taking blood from an artery, the state of blood flow is initially assessed. To do this, the artery is pressed and the degree of blanching of the distal area of the body is analyzed. If the blood flow is weak, other blood vessels are used to collect blood. Most often, blood is drawn from the arm.
After a 2 ml blood sample is taken, the puncture site is pressed for 5-10 minutes. It is imperative to take into account the high pressure present in the arterial bed.
Features of biomaterial collection
To assess the acid-base state of the body, blood is needed:
- Arterial.
- Venous.
- Capillary.
Arterial blood is the most suitable biomaterial for assessing gas composition. This is due to the fact that its study allows the most complete assessment of the degree of lung functioning.
Types of access:
- Radial artery puncture. The method is considered the simplest. After it is performed, the risk of developing a hematoma is less than 1%. A puncture of the radial artery is not performed if there is pronounced atherosclerosis in this area, as well as with a negative Allen test. The latter is carried out as follows: the patient must clench and unclench his fist several times until the skin of the hand turns pale, after which the vessel is pinched. If the natural color of the cover is restored in less than 5 seconds, this is considered normal. A longer process indicates a violation of blood flow.
- Femoral artery puncture. Disadvantages of the method: high risk of loss of liquid connective tissue, thrombosis, arm ischemia, vessel occlusion, infectious complications. Biomaterial collection is not carried out in the presence of a vascular prosthesis in this area, in case of aneurysm and local thrombosis, or taking anticoagulants. The complexity of the method lies in the fact that it is not always possible to puncture the artery on the first try.
The concentration of carbon dioxide, which is the end product of metabolism in tissues, is higher in venous blood. In this case, the amount of oxygen, on the contrary, is lower. If you analyze the acid base of venous blood, it becomes possible to evaluate the indicator of systemic metabolism. Collection is extremely rarely carried out from peripheral vessels, since the result of such a study is not clinically significant. The most common puncture is the pulmonary artery.
When collecting blood for acid-base balance (if it is carried out from a limb vessel), a tourniquet is never applied. This is explained by the fact that against the background of local blood circulation disturbance, the result of the study is significantly distorted and becomes uninformative.
If biomaterial is collected from a catheter installed in a central vein, the physician should avoid the channel through which electrolytes and glucose are administered. Blood acid-base level in such a case will also be considered uninformative due to falsely high values.
In terms of gas composition, capillary liquid connective tissue is closer to arterial tissue. Nevertheless, its analysis is considered the least informative. It is taken, as a rule, when it is necessary to assess the main indicators of the acid-base state of the blood in newborns.
Factors distorting the results
When taking a blood gas test, there are a number of factors that can cause distortion of the final results:
- increased or decreased body temperature;
- anemia or erythrocytosis - these pathologies impair the quality of oxygen that is carried along with the blood;
- immediately before the delivery of the biomaterial there was contact with tobacco.
Study of oxygen pressure status
The normal oxygen pressure also differs depending on age:
- adults – 4.7-6;
- children – 4.3-8.1.
When taking a blood gas test, this indicator may be within normal limits or may be reduced. In the latter case, the formation of hypoxia is diagnosed, which is accompanied by an increase or decrease in the amount of carbon dioxide.
Self-determination of acid-base status
It is important to understand that the information content of laboratory research is as high as possible. It is performed using modern automatic analyzers of blood gases, acid-base balance, electrolytes and glucose. If for some reason the patient needs to donate blood frequently, he can purchase a special device at the pharmacy and regularly use it to evaluate the pH value.
The principle of operation of the device is as follows: it has 2 electrodes, when placed in a drop of liquid connective tissue, an electromotive force is generated. After a minute analysis, the pH meter gives an accurate result.
The device can be analog or digital. In the first case, such models are considered outdated and are extremely rarely used by patients in practice. Digital pH meters are modern devices that not only have a user-friendly interface, but also a built-in prompt system and memory, so there is no need to record the result on paper every time.
In pharmacies and companies involved in the sale of medical equipment, you can purchase both stationary and portable devices. Thus, the patient can install a pH meter at home or buy a device that is not tied to a specific location and can be easily transported.
Carbon dioxide pressure indicator
The norm for such an indicator as carbon dioxide pressure also completely depends on age. In both cases the pressure is in the range from 35 to 45 mm.
If the studied indicator is less than 35 mm, then this indicates a violation of hyperventilation. There is a deficiency of carbon dioxide in the body. If the reading is more than 45 mm, an excess amount of carbon dioxide is diagnosed, which entails a decrease in heart rate and the development of a feeling of anxiety in the patient.
Indications for analysis
The first and most important reason is the diagnosis of respiratory failure, which allows us to identify disorders of the acid-base state of the blood, such as acidification - acidosis or alkalization, or alkalosis. Monitoring of blood gas composition is necessary for various serious diseases, for example, severe obstructive pulmonary lesions, idiopathic fibrosing alveolitis, and chronic renal failure. A blood gas test is also required for:
- monitoring patients over time with carbon monoxide poisoning;
- monitoring for the development of methemoglobinemia;
- in persons with low saturation.
This analysis is taken from patients on mechanical ventilation in intensive care units, and before surgery, this study is necessary to assess the risk of surgical intervention during thoracic operations on the lungs and chest organs, including the heart.
Bicarbonate indicator
Depending on the age of the patient, the following normal levels of bicarbonate are distinguished:
- adults – 22-28;
- children – 15-25.
If the value is below normal, this may be a sign of the development of renal pathologies, dehydration, or a metabolic form of acidosis. Exceeding the norm often develops with excessive use of steroids, hyperventilation and a metabolic form of alkalosis.