The request for "TORCH infection" is redirected here; see also other meanings.
| Intrauterine infections | |
| ICD-10 | 35.-39. |
| ICD-9 | 771 |
| MeSH | D018445 |
Intrauterine infections
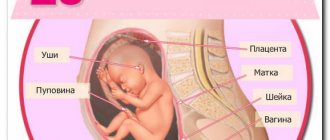
(IUI) are various infectious diseases of the embryo, fetus and newborn, infection of which occurs in utero and during childbirth [1]. The causative agents of infection can be viruses, bacteria and (less commonly) parasites. The route of transmission is vertical, from mother to fetus. The result of infection can be a miscarriage, congenital malformations, or an acute infectious process in a newborn.
Content
- 1 Epidemiology 1.1 TORCH
- 2.1 Source of infection
- 6.1 Neonatal herpes
Epidemiology
The true frequency of congenital infections has not yet been established, but, according to a number of authors, the prevalence of this pathology in the human population can reach 10%.
Intrauterine infections have the same patterns as infectious diseases in general.
They have a leading place in the structure of infant mortality.
The share of IUI in the structure of perinatal mortality in our country is almost 25%, however, transplacental infection of the fetus is considered one of the most likely causes of 80% of congenital malformations, which, in turn, account for about 30% of all deaths of children under 1 year of age [2 ][3]
TORCH
In 1971, WHO identified the concept of TORCH syndrome
.
This is an abbreviation for the most common intrauterine infections : T - toxoplasmosis, O - others, which includes mycoplasma, syphilis, hepatitis, streptococci, candida and other viral and bacterial infections, R - rubella (rubella), C - cytomegalovirus, H - herpes
If not clear etiological diagnosis, they talk about TORCH syndrome.
Intrauterine infection during pregnancy: what statistics say
- A timely diagnosed and treated infectious disease in a pregnant woman poses minimal danger to her child.
- Infectious agents pass from mother to baby in 10 out of 100 pregnancies.
- 0.5% of infants infected in the womb are born with corresponding signs of the disease.
- An infection that has settled in the mother’s body does not necessarily pass to the fetus, and the child has a chance to be born healthy.
- A number of infectious diseases that do not promise anything good for the baby may be present in the mother in a latent form and have virtually no effect on her well-being.
- If a pregnant woman gets sick with one or another infectious disease for the first time, there is a high probability that her child will also become infected.
Etiology
An infectious process in the fetus can be caused by a wide variety of pathogens. According to this principle, several groups can be distinguished.
- A group of IUIs caused by viruses: rubella, CMV, herpes viruses, viral hepatitis, etc.
- Diseases caused by bacteria: syphilis, listeriosis, tuberculosis, STDs
- Parasitic infections: toxoplasmosis
- Fungal infections, including iatrogenic origin
- Mixed infections (combined).
It is noteworthy that infection with the same infections in the postneonatal period occurs in most cases asymptomatically or in the form of a mild infectious process. The causative agents of infectious diseases that the mother first encountered during pregnancy are especially dangerous for the fetus, since during this period the primary immune response is reduced, while the secondary one is normal.
Source of infection
The source of infection is the mother. But there are also iatrogenic causes of infection during medical procedures [4] [5].
Routes of infection
- Transplacental (hematogenous) route - from mother to fetus through the placenta. Viral IUIs are more often transmitted, since the virus easily penetrates the blood-placental barrier (like Toxoplasma).
- Ascending - when an infection from the genital tract enters the uterine cavity and can then infect the fetus. More often these are bacterial infections, STDs, chlamydia, fungi, mycoplasmas, enterococci.
- Descending path - from the fallopian tubes into the uterine cavity
- Contact (intranatal) route - infection during passage through the birth canal.
Outcome of fetal infection
- Infection,
- Sanitation of the pathogen with the acquisition of immunity,
- Carriage of an infectious agent with the possibility of developing the disease in the future. Thus, the presence of an infection in the mother, an infectious lesion of the placenta and infection do not mean 100% development of IUI in the fetus and newborn[1]
Examples of decoding analysis for TORCH infection
When reading the form with the results of an antibody test, an interested person can see some numerical values - how can one understand where –lgG/lgM, +lgG/lgM and +lgG/lgM are?
Let's pay attention to the analysis form - we see the column “reference values”. This column is divided into three parts - “negative”, “weak positive” and “positive”. The result of the detected antibody is displayed in some numerical values, units IU/ml (international unit per milliliter).
The amount of detected antibody correlates with the reference limits indicated on the form of numerical values, falling into one or another part of the column. Below, in the example, we have indicated with red arrows all +lgG for clarity.
We similarly arrange the values for each antibody to each of the TORCH infections. We add the resulting lgG and lgM together and read the result in accordance with the decoding:
A) Cytomegalovirus
B) Herpes
B) Rubella
Important! All this data is presented to familiarize yourself with the general principles of generating analysis results for TORCH infections. In no way can the information presented here replace consultation with a doctor! Only a specialist can correctly decipher the results of such testing, take into account all the nuances and make the correct diagnosis.
Symptoms
All IUIs have a number of common symptoms. The similarity of symptoms is associated with several points: the characteristics of the pathogens are often intracellular infections, the body cannot independently eliminate infections - as a result, a persistent course. In addition, newborns have age-related weakened immunity, which is why infections take a slow course. As a result of the effect of infection on the fetus, a complex of effects occurs, such as hyperthermia, the pathological effect of microorganisms and their toxins, resulting in a disruption of the placentation process and metabolic disorders [6]. [7]
- Manifestations of infection are determined by the timing of infection of the fetus in the first 2 weeks after conception - blastopathy, which often ends in spontaneous abortion at a very early stage.
- from 2 to 10 weeks of pregnancy - true malformations due to lesions at the cellular level.
- from 10 to 28 weeks of pregnancy - early fetopathies. The fetus can respond to the introduction of an infection with a generalized inflammatory reaction (the 1st and 3rd phases of inflammation, alteration and proliferation and fibrosis are clearly expressed, and the 2nd phase - exudation is not pronounced) as a result of which the child develops multiple malformations, for example fibroelastosis.
- from 28 to 40 weeks of pregnancy - late fetopathies. The fetus can already respond with a full-fledged inflammatory reaction, most often several organs are involved
- infection during childbirth - inflammation, more often than one organ - pneumonia, hepatitis.
General signs[6]:
- intrauterine growth restriction
- hepatosplenomegaly
- minor developmental anomalies (stigmas of disembryogenesis) early or prolonged or intense jaundice
- rashes of various types
- respiratory distress syndrome
- cardiovascular failure
- severe neurological disorders
- febrile conditions in the first days of life
Causes of intrauterine infection during pregnancy
The activity of ubiquitous pathogenic microorganisms is the main cause of morbidity among women who are preparing to become mothers. Many bacteria and viruses, entering the mother's body, are transmitted to the child, provoking the development of serious anomalies. Viruses responsible for the development of acute respiratory viral diseases do not pose a danger to the fetus. A threat to the child’s condition appears only if a pregnant woman develops a high body temperature.
One way or another, intrauterine infection of the baby occurs exclusively from the sick mother. There are several main factors that can contribute to the development of infectious pathology in the fetus:
- Acute and chronic diseases of the mother in the genitourinary system. Among them are such inflammatory pathologies as cervical ectopia, urethritis, cystitis, and pyelonephritis.
- The mother has an immunodeficiency state or HIV infection.
- Organ and tissue transplantation that the woman has undergone in the past.
Diagnostics
Diagnosis of IUI includes two mandatory components: 1) clarification of the nature (etiology) of the infection and 2) proof of the intrauterine genesis of the disease. Diagnosing IUI is extremely difficult. Anamnesis data and features of the course of pregnancy can only suggest the possibility of intrauterine infection. Accurate diagnosis involves examining 1) the mother, 2) the placenta, and 3) the fetus (newborn, child). The study of the placenta (placenta, membranes and umbilical cord) must be of high quality, which involves studying at least 2 pieces of the umbilical cord, 2 rollers of the membranes (twisted from the site of rupture to the place of attachment to the placenta) and 10 pieces of the placenta. It is necessary to conduct bacteriological and immunohistochemical (IHC) studies of the placenta and membranes. The introduction of IHC studies into the practice of a pathologist is absolutely necessary. This is the only way to overcome the existing overdiagnosis of chlamydia, mycoplasmosis, toxoplasmosis, “deenkova” and other infections. The immunofluorescence method when examining the placenta gives a large number of false positive results. Methods for laboratory diagnosis of IUI can be divided into direct and indirect.
Direct ones include:
- microscopy
- culture method, virus replication on tissues
- Detection of antigens by RIF, ELISA and IHC.
- PCR[8]
Indirect diagnostic methods are serological studies using enzyme-linked immunosorbent assay (ELISA), qualitative and quantitative analysis of IgM, IgG, IgA. The newborn's blood is examined. The presence of IgG may indicate transplacental transfer of maternal antibodies, so the newborn’s blood is tested again after 3-4 weeks. An increase in IgG titer of 4 times or more is diagnostically significant[9]. The detection of IgM in the blood of a newborn indicates the presence of an active infection in the child. From additional studies, a general blood test can detect leukocytosis with a shift to the left, leukocytosis with neutropenia, toxic granularity of neutrophils, anemia. In addition, children with suspected IUI need to undergo abdominal ultrasound to detect hepatosplenomegaly and neurosonography [10] [11].
Which doctors to visit
Of course, during pregnancy a woman should carefully monitor her health. Therefore, in addition to the tests listed, you will have to regularly visit certain doctors. Which ones? Now we will figure this out.
First on our list is the dentist. It is advisable to treat all teeth before pregnancy. Otherwise, therapy may be delayed. It’s not particularly pleasant to sit in line every month to see such a “scary” doctor.
Next you need to visit a therapist and an ophthalmologist. The ophthalmologist, by the way, will have to issue a conclusion on the birth in one form or another: caesarean section or natural way.
A cardiologist and a dermatologist are two other doctors who are recommended to visit. However, some try to refrain from such visits. After all, a pregnant woman already visits many different doctors. If you have a strong heart and no allergies, then you can ignore these doctors.
Clinical forms
Neonatal herpes
| IUI: neonatal herpes | |
| ICD-10 | 35.235.2 |
| ICD-9 | 771.2771.2, 054.xx054.xx |
| MeSH | D018445 |
Main article: Herpes
Of the herpes family viruses, all the main types can cause herpes infection in a newborn: herpes simplex virus types 1 and 2, herpes simplex virus type 3 (varicella zoster), type 4 - Epstein-Barr virus, hairy leukoplakia of the tongue, immune depression syndrome, 5- 1st type - cytomegalovirus infection, 6th type - roseola, 7th type - chronic fatigue syndrome, 8th type - Kaposi's sarcoma. However, the term “neonatal herpes” is used only in relation to diseases caused by herpes simplex virus types 1 and 2. The most dangerous for a child is HSV-2.
The likelihood of a child becoming infected depends on how long the mother has been infected. The “fresh” the infection, the more likely the child is to become infected. If at the time of birth the mother has a rash, this is an indication for a cesarean section[12].
Clinical manifestations
- 1. Local form (mucocutaneous) - damage to the skin, mucous membranes, and, less commonly, encephalitis.
- 2. Cerebral form - the clinical picture corresponds to the general signs of IUI, but there are also specific signs: damage to the eyes and mucous membranes; herpetic encephalitis, which is necrotic in nature, resulting in destruction of the brain down to the hemispheres; severe thrombocytopenia with hemorrhagic syndrome.
- 3. Disseminated neonatal herpes
Diagnosis
In the diagnosis of neonatal herpes, assessment of the mother's specific medical history is important. During a clinical examination of children born to mothers with acute or recurrent genital herpes, examination of the skin and mucous membranes must be done with special care. If a newborn has seizures of unknown etiology, a lumbar puncture is indicated (with herpetic encephalitis, lymphocytosis, monocytosis and a high protein concentration are noted). If a newborn develops sepsis and there is no effect of antibiotics, an examination for herpes is necessary. Among laboratory diagnostic methods, the gold standard is the isolation of the virus from blood, cerebrospinal fluid, and vesicles by the cultural method. In the cutaneous form, the contents of the vesicles or scrapings from the skin can be examined using the immunofluorescent method to detect the virus antigen. And in case of generalized infection and meningoencephalitis, blood and cerebrospinal fluid are examined using the PCR method. The level of IgG antibodies is not informative, since these are maternal antibodies. IgM levels indicate acute infection in the newborn.
Treatment
For all forms of neonatal herpes, systemic antiviral therapy is indicated, since the localized form may precede the generalized one. With early administration of antiviral drugs, the outcome is favorable. Regardless of the form of infection, acyclovir is used. Acyclovir (Zovirax, Virolex) intravenously for 2-3 weeks plus antiherpetic immunoglobulin for 2 weeks[12]. It makes no sense to stop breastfeeding, since HSV is unlikely to pass into mother's milk, except for rashes on the mother's breasts. Local remedies for ophthalmic herpes include vidarabine, florenal, and bonaftone ointment.
Congenital cytomegalovirus infection
Main article: Cytomegalovirus
The frequency of occurrence is 0.2-2.5%. The virus is transmitted by all secretions (saliva, urine, blood, tears). Clinical manifestations during primary infection in pregnant women are nonspecific and may resemble the clinical manifestations of acute respiratory viral infection. There are a number of factors contributing to the high incidence of intrauterine infection with cytomegaly virus. These include epidemiological features, such as significant genetic variability of CMV strains, the widespread distribution of CMV infection in the human population (in the vast majority - in the form of a latent-persistent course), the predominance of subclinical forms, both in primary and secondary infection, diversity mechanisms and routes of transmission of infection. The next factor is the immaturity of the immune system of the fetus and newborn. And finally, adaptive immune changes in a woman’s body during pregnancy (decreased functional activity of cellular immune mechanisms), during which reactivation of a latent-persistent CMV infection is possible.
Infection most often occurs during childbirth, or through mother's milk. During pregnancy, infection occurs only if the mother becomes infected for the first time during pregnancy.
Clinical manifestations
Congenital hepatitis with severe jaundice, severe thrombocytopenia with hemorrhagic syndrome, meningoencephalitis. Specific signs are calcifications in the subependymal parts of the brain and chorioretinitis. The long-term prognosis is determined by the degree of brain damage. If meningoencephalitis is suffered early, children are usually disabled; if hepatitis, cirrhosis develops early; if carditis, chronic heart failure develops.
Diagnostics
Children with symptoms of congenital infection, as well as without clinical manifestations of TORCH syndrome, if they are born to women at risk, are subject to examination for CMV infection. In newborns in the early neonatal period, if CMV is suspected, the pathogen is first identified by any available method. Most often, PCR or detection of virus antigens is used; the virological method is less often used. Any biological fluid (urine, saliva, blood, tears) can serve as material for PCR; however, active CMV infection is indicated only when the CMV genome is detected by PCR in the blood and cerebrospinal fluid. When viral DNA is found in other environments, an unambiguous assessment of the period of the disease cannot be given. To clarify the severity of the process, serological methods are used - anti-cytomegalovirus antibodies of classes M and G are determined. Moreover, the study of “paired sera” is mandatory, that is, monitoring the study of antibody titers after 3-4 weeks. Detection of IgM class antibodies in umbilical cord blood and in the blood of a child in the first weeks of life is an important diagnostic sign. And the detection of IgG in the child’s blood without comparison with maternal titers is not diagnostically significant, since transplacental transfer of antibodies from the mother’s body is possible.
Treatment
Therapy for congenital CMV infection consists of etiotropic and syndromic therapy. The indication for etiotropic therapy is the active period of congenital CMV infection. The drug of choice for etiotropic treatment is Cytotect. Children are administered anticytomegalovirus immunoglobulin (cytotect) intravenously 2 ml/kg 2 times a day every 2 days for 3 weeks [13].
If there is a danger to life, then ganciclovir is added intravenously for 14-21 days, although virostatics (antiviral drugs) such as ganciclovir and foscarnet are used extremely rarely due to their high toxicity.
Congenital toxoplasmosis
Main article: Toxoplasmosis
Frequency 1:1000 newborns Toxoplasma oocysts are usually found in the feces of cats and goats, from where they are released into the external environment. In pregnant women, the clinical course of the disease is similar to mononucleosis or influenza, with high fever or very long-term low-grade fever, and enlarged lymph nodes. Arthralgia or arthritis is often associated.
The likelihood of infection of the fetus: infection usually occurs if the infection is fresh and depends on the duration of infection. If the 1st trimester - the probability is 15%, in the second 30%, in the third - 60%.
Clinical manifestations
In the fetus and newborn, the infection can take two forms: damage to the eyes and brain or generalized toxoplasmosis. In addition to the general signs of infectious toxicosis, hepatitis, meningoencephalitis, eye damage (congenital cataract, possibly glaucoma, optic nerve atrophy) are added.
Diagnostics
Scheme of examination for toxoplasmosis of newborns: in the presence of clinical signs of toxoplasmosis, antibodies are examined. If no antibodies are detected, the test is repeated after 2 weeks; if there are no antibodies during the second test, then further monitoring is not needed. If detected, specific therapy is indicated. If IgM class antibodies are detected during the initial study, then etiotropic therapy is immediately indicated. If only IgG is detected, the test is repeated after 4 weeks. Therapy is indicated when the antibody titer increases. If the titer drops, the child does not need treatment, but further monitoring is necessary.
Treatment
Treatment of toxoplasmosis can be carried out antenatally - that is, treatment of a pregnant woman. If the infection is in the 1st half of pregnancy, spiramycin, claforan, rovamycin are used. If in the 2nd half of pregnancy - chloridine + sulfasalazine + folic acid. Treatment of children is effective during periods of circulation in the blood of non-cystic forms of the parasite; drugs do not act on cystic forms. There is no need for complete sanitation, since cystic forms (carriage) provide normal non-sterile immunity. The most effective drugs are pyrimethamine in combination with sulfonamides. There are combination drugs: Fansidar, Metakelfin. Co-trimoxazole is also used in age-specific dosages[14]. Treatment of newborns involves the following regimen: chloridine + sulfadimezine + folic acid. Course 4-6 weeks. During the first year, 4 courses with a break of 1.5 months, and during the break, spiramycin for 1.5 months [12][15][16].
Chlamydia
Main article: Chlamydia
WHO data indicate that 35-50% of newborns whose mothers are infected with C. trachomatis
, chlamydial ophthalmia develops (5 times more often than gonococcal ophthalmia), and in 11-20% pneumonia develops[17].
Infection usually occurs during childbirth, the probability of transmission is 40-70%. The disease does not appear immediately, but after 7-14 days. Clinical manifestations
There are three forms of infection in newborns:
- persistent
- latent
- acute (generalized infection - meningoencephalitis, intrauterine pneumonia, gastroenteritis)[17][18].
The main manifestations of the disease in a newborn are:
- nasopharyngitis 25%
- conjunctivitis resistant to the use of conventional remedies, responds only to treatment with tetracycline ointment,
- pneumonia 10-15% - mild toxicosis, but pronounced obstructive syndrome, paroxysmal painful cough.
- high eosinophilia
- proctitis, gastroenteritis - 5%
- vulvitis, urethritis 15%
Treatment
In addition to the child, it is necessary to treat both the father and mother. A newborn child is prescribed erythromycin in suppositories for 24 days or erigran orally. Azithromycin can also be used[12][19].
Mycoplasmosis
Main article: Mycoplasmosis
Mycoplasma infection usually occurs during childbirth. The frequency of detection of the pathogen in pregnant women is 20-50%, the risk of infection of the fetus is unknown. Pregnant women with seropositive mycoplasmosis are treated after the 16th week of pregnancy, which reduces the incidence of morbidity in newborns.
Clinical manifestations
In newborns, it manifests itself in the form of pneumonia, which begins imperceptibly with toxicosis, pallor appears, shortness of breath increases, and only then physical findings appear. On the radiograph, a specific sign is the “blizzard symptom” - bilateral fine-focal, sometimes confluent pneumonia. Mortality rate is 15%.
Treatment
Newborns are prescribed erythromycin or azithromycin, and in severe forms, chloramphenicol [20][12].
Congenital rubella syndrome
| Intrauterine infections | |
| Cataract in congenital rubella syndrome | |
| ICD-10 | 35.035.0 |
| ICD-9 | 771.0771.0 |
| DiseasesDB | 11729 |
| MedlinePlus | 001658 |
| eMedicine | emerg/388 |
| MeSH | D012410 |
Main article: Rubella
If the mother becomes infected in the first 12 weeks, it is better to terminate the pregnancy. Before pregnancy, it is necessary to be examined, and if the mother is seronegative, then vaccinated[12]. If a mother becomes infected with rubella in the 1st trimester, the child has a 25% chance, after the 5th month – 1-2%.
Clinical manifestations
A characteristic clinical manifestation is Greg's triad
:
- congenital heart disease such as patent ductus arteriosus, ventricular septal defect, atrial septal defect, pulmonary stenosis, myocardial necrosis
- eye defects (cataracts, microphthalmia, glaucoma)
- deafness.
In 2/3 children, congenital rubella manifests itself at the end of the perinatal period.
Treatment
There is no specific therapy; treatment is symptomatic.
Candidiasis of newborns
Main article: Candidiasis
The frequency of candidiasis in the structure of infectious and inflammatory diseases of newborns is about 15-30% of cases, and in half of them it remains unrecognized or diagnosed late[21]. Candidiasis can be caused by any of the species, but most often by Candida albicans. Risk factors for the development of candidiasis in newborns include: prematurity, diabetes mellitus in the mother during pregnancy, urogenital candidiasis in the mother during pregnancy, repeated courses of antibiotics, especially in combination with immunosuppressive therapy, immune disorders, especially neutropenia, the presence of mechanical ventilation in the early neonatal period, resuscitation measures, abdominal operations.
Clinical manifestations
Based on the time of infection, congenital
candidiasis that developed during antenatal or intrapartum infection and
postnatal
candidiasis. Depending on the localization of the process, candidiasis is divided into:
- cutaneous candidiasis is a lesion of the skin and its appendages. The lesion may be localized or widespread.
- candidiasis of the mucous membranes of the oral cavity, conjunctiva, external genitalia
- generalized candidiasis - damage to internal organs that do not communicate with the external environment with the addition of candidemia.
- visceral candidiasis - damage to internal organs and other systems that do not communicate with the external environment, for example carditis, hepatitis, nephritis.
- systemic candidiasis - affects one or more organs that communicate with the external environment - for example, candidiasis of the gastrointestinal tract.
- candidiasis - the presence of Candida in natural habitats in greater than the prescribed concentration.
Candidiasis is also classified according to the severity of the process as mild.
and
severe
forms, depending on the location and volume of the lesion, the presence of infectious toxicosis.
In addition, there are acute
(7-14 days) and
protracted
(more than 6 weeks) course of the disease.
Diagnosis
Diagnosis of neonatal candidiasis is based on the clinical picture. In the cutaneous and mucocutaneous form there is no need for laboratory confirmation. Laboratory diagnosis becomes crucial for generalized, visceral and systemic candidiasis. Laboratory criteria can be considered the identification of fungi in an active state by microscopy of the substrate, the isolation of antigens and DNA in sterile substrates, the isolation in quantities greater than those allowed for sowing of substrates that are the site of saprotification of fungi.
Treatment
For localized skin candidiasis, local therapy with antifungal ointments (clotrimazole, isoconazole, ketoconazole, natamycin) is used. In case of prolonged course, systemic antimycotics are prescribed - fluconazole orally. The daily dose is 5-8 mg/kg once a day. For mucosal candidiasis, the affected areas are treated with a 2% soda solution or a 0.1% hexoral solution. In case of relapse, fluconazole is used. For systemic candidiasis for the treatment of the gastrointestinal tract, respiratory, genitourinary system, as well as for visceral and generalized candidiasis, treatment begins with fluconazole, and if ineffective, amphotericin B or ambisome is prescribed intravenously for 5-7 days[22].
Early congenital syphilis
Main article: Syphilis
Against the backdrop of an epidemic increase in the incidence of syphilis in Russia in the 90s, the incidence of congenital syphilis increased sharply: in 1997, the overall incidence of syphilis exceeded the level of 1990 by 51 times, and that of congenital syphilis by 47 times. The dynamics of the increase in the incidence of congenital syphilis is similar to the dynamics of the proportion of pregnant women among women with syphilis. According to L.I. Tikhonova (1999), in 1995-97. in Russia this figure was constantly growing: 4.9%, 5.5%, 6.5%[23].
Congenital syphilis can be prevented by identifying and treating infected mothers during pregnancy. Therefore, pregnant women are examined serologically three times, including immediately before birth.
Early congenital syphilis is an IUI that manifests itself in a child under 2 years of age (according to ICD-10). Early congenital syphilis may be manifest
(with clinical manifestations) and
hidden
.
Clinical manifestations
In newborns with early congenital syphilis, the following symptoms are observed: syphilitic rhinitis, diffuse Hochsinger infiltration, chorioretinitis, hepatosplenomegaly, syphilitic pemphigus, roseolous and pustular rash, osteochondritis, periostitis, osteoporosis.
Diagnostics
In newborns born to mothers with syphilis, umbilical cord blood is taken for analysis at birth to carry out a set of serological tests. In addition, weighing and pathological examination of the placenta are carried out. With syphilis, the placenta is enlarged and there are signs of inflammation. A spinal tap must be performed. In the analysis of cerebrospinal fluid there are specific changes: lymphocytic cytosis above 20 cells in 1 ml, protein above 1.5-1.7 g/l, positive results of RIF and a complex of serological reactions. On the 7-8th day of the child’s life, serological blood tests are repeated - microprecipitation reaction, immunofluorescence reaction, immobilization reaction of Treponema pallidum, enzyme immunoassay to detect IgM.
Treatment
Treatment is carried out with one of the penicillin drugs for 2-3 weeks. The choice of drug depends on the analysis of the cerebrospinal fluid. After completion of treatment, the child is discharged under the supervision of a dermatovenerologist, and the diagnosis is reported to the district clinic only with the consent of the mother. In the KVD, clinical and serological control is carried out once every 3 months until the child reaches the age of 3 years[24].
Notes
- ↑ 1 2 V.V. Vlasyuk
Morphological diagnosis of intrauterine infections. Tutorial. St. Petersburg, 2010 - 47 p. ISBN - 5-00-001976-8/ - Okhotnikova I.M., Ageikin V.A., Lozovskaya L.S. The significance of intrauterine viral infection in organ pathology of infants // Med. scientific and educational-methodological. magazine. - 2001. - No. 5. - P. 81-87.
- Congenital malformations. Prenatal diagnosis and tactics / Ed. B. M. Petrikovsky, M. V. Medvedev, E. V. Yudina. - M.: RAVUZDPG: Realnoe Vremya, 1999. - 325 p.
- https://spravka.komarovskiy.net/vnutriutrobnye-infekcii.html Komarovsky. Intrauterine infections
- https://www.ic.omskreg.ru/~medstat/BIBLIO/opport/index.htm Dolgikh T. I., Noskova F. V. Opportunistic infections in children (issues of diagnosis, clinic and treatment). Omsk: Publishing house OGMA, 1999. - 99 p.
- ↑ 12
A. L. Zaplatnikov, N. A. Korovina, M. Yu. Korneva, A. V. Cheburkin A. L. Zaplatnikov, N. A. Korovina, M. Yu. Korneva, A. V. Cheburkin Attending physician 08- 2005 https://www.lvrach.ru/doctor/2005/08/4532901/ - A. Ya. Senchuk, Z. M. Dubossarskaya.
Perinatal infections: practical. allowance. - M.: MIA, 2004. - 448 p. - Diagnosis of intrauterine infections in newborns using the polymerase chain reaction method
- Intrauterine infections. Symptoms. Diagnostics. Prevention. | EUROLAB | Pediatrics
- Volodina N. N., Degtyareva D. N.
Diagnosis and treatment of intrauterine infections. - M.: Method. rec. for neonatologists, 1999. - https://www.rae.ru/ru/publishing/mono07_811.html MODERN PRINCIPLES FOR DIAGNOSIS OF INTRAuterine INFECTION OF THE FETAL
- ↑ 123456
Perinatal infections (issues of pathogenesis, morphological diagnosis and clinical and morphological comparisons) Tsinzerling V. A., Melnikova V. F. - Clinical and laboratory characteristics, pathomorphological features, diagnosis and treatment of cytomegalovirus pneumonia “Infectious Diseases”, 2004. - T. 2, No. 1. - P. 73-80. V. I. Shakhgildyan, O. A. Tishkevich, O. Yu. Shipulina. https://www.hivrussia.ru/pub/2006/16.shtml
- Cheburkin A.V. Clinic and differential diagnosis of congenital toxoplasmosis “Medical parasitology and parasitic diseases” No. 5, 1984, p. 53-57.
- On the detection and prevention of toxoplasmosis in Moscow. Methodological recommendations (No. 25). M., 2007
- Clinic, diagnosis and treatment of toxoplasmosis G. Yu. Nikitina, F. K. Dzutseva, Yu. V. Borisenko, L. P. Ivanova Attending physician 10-2008 https://www.lvrach.ru/doctore/2008/10/ 5828652/
- ↑ 12
Urogenital chlamydia Chlamydial Genitourinary Infections https://www.venuro.info/venera/chlamidioz.php - Chlamydia Uskov Alexander Nikolaevich https://queerlvov.narod.ru/hlamidioz.html
- https://www.hlamidioz.info/6.html Chlamydia in children
- Perinatal infections: practical. manual / ed. A. Ya. Senchuk, Z. M. Dubossarskaya. M.: MIA, 2005. 318 p.
- Protocols for diagnosis, treatment and prevention of intrauterine infections in newborns, Moscow, State Educational Establishment VUNMC Ministry of Health of the Russian Federation, 2001 p. 53
- Protocols for diagnosis, treatment and prevention of intrauterine infections in newborns, Moscow, State Educational Establishment VUNMC Ministry of Health of the Russian Federation, 2001 pp. 55-57
- https://www.ill.ru/news.art.shtml?c_article=142 Syphilis and pregnancy
- Protocols for diagnosis, treatment and prevention of intrauterine infections in newborns, Moscow, State Educational Establishment VUNMC Ministry of Health of the Russian Federation, 2001 pp. 59-64
additional literature
- Degtyarev D.N., Degtyareva M.V., Kovtun I.Yu., Shalamova L.V.
Principles of diagnosing intrauterine infections in newborns and tactics for managing children at risk. - M.: Perinatology today, 1997. - T. 3. - P. 18-24. - Volodina N. N., Degtyareva D. N.
Diagnosis and treatment of intrauterine infections. - M.: Method. rec. for neonatologists, 1999. - Cheburkin A.V., Cheburkin A.A.
Perinatal infection. - M., 1999. - N. N. Volodin.
Current problems of neonatology. - M.: GEOTAR-MED, 2004. - 448 p. - A. Ya. Senchuk, Z. M. Dubossarskaya.
Perinatal infections: practical. allowance. - M.: MIA, 2004. - 448 p.