Types of hemostasis
The blood coagulation system consists of three main components:
- the coagulation system itself - prevents and eliminates blood loss;
- anticoagulant system - prevents the formation of blood clots;
- fibrinolysis system - dissolves already formed blood clots.
- platelet hemostasis - ensured by the adhesion (gluing) of platelets;
- coagulation hemostasis - is ensured by special plasma proteins - factors of the blood coagulation system.
All these three components must be in constant balance to prevent blockage of blood vessels with blood clots, or, conversely, high blood loss.
Hemostasis, that is, stopping bleeding, is of two types:
Coagulation
Coagulation or blood clotting is the body's defense mechanism against large blood loss. Today, approximately half of humanity has problems with coagulation. It is because of them that such terrible diseases as thrombosis, heart attack, stroke, and extensive bleeding occur. Every tenth person dies as a result of untimely treatment of these blood pathologies, and every second person does not even suspect that he has a coagulation disorder.
Coagulation is a sequential series of processes, each of which triggers the next. If there is a failure at any stage of coagulation, a pathology occurs that prevents normal blood clotting. Today, scientists have identified the main phases of blood clotting, these are:
- The appearance of prothrombin.
- The appearance of thrombin.
- Fibrin activation.
The last phase of stopping bleeding is the narrowing and dissolution of the blood clot, which turns into an initially liquid state.
Platelet hemostasis
This type of bleeding stop is the first to be activated, even before the coagulation is activated. When a vessel is damaged, its spasm is observed, that is, a narrowing of the lumen. Platelets are activated and adhere to the vascular wall, which is called adhesion. Then they are glued together and fibrin threads. Their aggregation occurs. At first this process is reversible, but after the formation of a large amount of fibrin it becomes irreversible.
This type of hemostasis is effective for bleeding from small-diameter vessels: capillaries, arterioles, venules. To finally stop bleeding from medium and large vessels, activation of coagulation hemostasis, provided by blood coagulation factors, is necessary.
Why does blood clotting occur?
The process of blood clotting is a protective reaction that prevents excessive blood loss when blood vessels are damaged and maintains a relatively even blood volume in the body. Normally, the process of thrombosis starts when a change occurs in the physical and chemical composition of the blood. The basis of this process is dissolved fibrin, which during bleeding turns into an insoluble form.
The protein in the area of injury forms a network, resembling thin threads, which traps blood cells, forming a clot. As the blood clot thickens, it causes the edges of the injury to tighten.
Coagulation hemostasis
This type of bleeding control, unlike the platelet type, comes into play somewhat later; more time is needed to stop blood loss using this method. However, it is this hemostasis that is most effective for the final stop of bleeding.
Clotting factors are produced in the liver and circulate in the blood in an inactive form. When the vessel wall is damaged, they are activated. First of all, prothrombin is activated, which is then converted into thrombin. Thrombin breaks down large fibrinogen into smaller molecules, which at the next stage combine again into a new substance - fibrin. First, soluble fibrin becomes insoluble and provides final cessation of bleeding.
Main components of coagulation hemostasis
As noted above, the main components of the coagulation type of bleeding control are clotting factors. There are 12 of them in total, each of which is designated by a Roman numeral:
Previously, factor VI (accelerin) was also present in the classification, but it was removed from the modern classification, since it is the active form of factor V.
In addition, one of the most important components of coagulation hemostasis is vitamin K. Some coagulation factors and vitamin K are directly related, because this vitamin is necessary for the synthesis of factors II, VII, IX and X.
Treatment of hereditary clotting factor deficiency
According to existing standards, treatment is carried out with vitamin K preparations (orally, parenterally), complex prothrombin concentrate (CPC) prescribed intravenously and fresh frozen plasma (FFP) administered intravenously. The best effect is achieved by infusion of FFP, which contains all clotting factors. The only negative point is the increase in circulating blood volume, which determines the need to control hemodynamic parameters. It is best to use virally inactivated FFP obtained from multiple donors.
Essential drugs
There are contraindications. Specialist consultation is required.
| 1 | Complex prothrombin concentrate (Prothromplex 600) is a preparation of plasma factors (II, VII, IX, X) of blood coagulation. |
Dosage regimen: administered intravenously. Therapy begins under the supervision of a specialist with experience in correcting disorders of the hemostatic system. The single dose and frequency of administration are determined individually. The dose is calculated based on INR values as follows:
| Initial MHO value | Single dose of the drug per 1 kg of body weight |
| 2,0-3,9 | 25 IU/kg |
| 4,0-5,9 | 35 IU/kg |
| ≥ 6,0 | 50 IU/kg |
The issue of administering a second dose of the drug is decided individually based on the results of the achieved INR value and clinical dynamics.
| 2 | Vitamin K (Vikasol) is an antihemorrhagic agent. |
Dosage regimen: adults orally - 15-30 mg/day, intramuscularly - 10-15 mg/day. Children under 1 year old - 2-5 mg/day, up to 2 years old - 6 mg/day, 3-4 years old - 8 mg/day, 5-9 years old - 10 mg/day, 10-14 years - 15 mg/day. Frequency of administration: 2-3 times/day. The duration of therapy is determined individually. With long-term use of Vikasol, take a 4-day break every 3-4 days.
Main types of factors
The 12 main components of coagulation hemostasis listed above are plasma coagulation factors. This means that these substances circulate freely in the blood plasma.
There are also substances that are located in platelets. They are called platelet-derived clotting factors. Below are the main ones:
The same compounds have been discovered in other blood cells: erythrocytes and leukocytes. During hemotransfusion (blood transfusion) with an incompatible group, massive destruction of these cells occurs and platelet-derived coagulation factors come out in large quantities, which leads to the active formation of numerous blood clots. This condition is called disseminated intravascular coagulation syndrome (DIC).
Types of blood clots
There are four types of blood clots.
- White. The basis of the clot is fibrin, platelets and leukocytes. There are minimal red cells in the clot. Formed mainly in arteries.
- Red. The composition of a blood clot is red blood components, fibrin and platelets. Formed in veins.
- Mixed type. The most common form of thrombus, in which the composition contains approximately equal proportions of components from the first two types. Formation is possible in the veins, heart and aortic aneurysm.
- Hyaline. The clot contains hyalized red blood cells and blood plasma proteins. There is practically no fibrin in the composition. Clots form in the capillaries.
The type of thrombus that forms depends on the type of damaged vessel.
Types of coagulation hemostasis
There are two mechanisms of coagulation: external and internal. Tissue factor is required to activate the external factor. These two mechanisms converge to produce coagulation factor X, which is necessary for the formation of thrombin, which in turn converts fibrinogen into fibrin.
The cascade of these reactions is inhibited by antithrombin III, which is able to bind all factors except VIII. Coagulation processes are also influenced by the protein C - protein S system, which inhibit the activity of factors V and VIII.
Platelet-derived clotting factors
All of them are located inside platelets, or blood platelets, whose task is to deal with hemostasis, or all processes associated with blood clotting. Platelets can also be active or inactive. In the blood they are most often found near the vascular wall, and are rarely in the center of the blood flow. This is understandable, since their task is to monitor the integrity of the vascular wall, and immediately engage in the coagulation process if a threat of damage is detected.
We will not list all 11 factors in detail, we will only say that among them there are several identical to plasma ones. For example, there is a platelet analogue of fibrin, or proaccelerin. When platelets are activated, then they, in turn, in addition to these 11 factors, begin to secrete many protein substances, ions, and enzymes. More than two dozen such substances are now known, including platelet fibronectin, heparinase, plasminogen activator, and even serotonin. On the appropriate resources, you can carefully study the table of platelet coagulation factors. How is the complex, multiply duplicated hemostasis system organized?
Two ways of blood clotting, or “how does it all work”?
It turns out that there is no single path of hemostasis in the human body. Conventionally, there are two types of blood clotting:
The first path is called external, when there is some damaging factor from the outside, and it injures the vessel.
This is any open wound, traumatic injury. In this case, thrombin is formed to block the vessel and stop bleeding;
Along with the external pathway, there is also a more mysterious and less studied internal pathway for the formation of the same thrombin, when no injury occurs, the vascular wall does not violate its integrity, and the coagulation factors of which it is the source are absent.
This internal path can be realized for a very large number of reasons, including a variety of diseases and pathological conditions. Both pathways are united by the presence of calcium ions and special phospholipid structures in the cascades of these reactions.
For the normal, extrinsic pathway, the source of phospholipids is tissue thromboplastin. It is always found in tissues destroyed by injury. When it mixes with the blood, the cascade of the external hemostasis pathway begins to start. In order for external hemostasis to proceed correctly, the participation of VII, IX, X, and II plasma coagulation factors, which were mentioned above, is necessary.
If we talk about the internal path of hemostasis, then there will no longer be tissue thromboplastin as a source of phospholipids, since the tissues are not destroyed. Then the donors of these phospholipids become blood platelets - platelets, and vascular endothelial cells. There are activators here, namely factors XI and XII. The role of damaged and destroyed tissues here are played by their analogues, and the body, unable to recognize where the true destruction of the tissue is and where the false one, triggers the internal cascade of hemostasis in roundabout ways. What are these structures that simulate the destruction of tissues and blood vessels?
These can be various foreign surfaces, for example, heart valves. Quite often these are circulating immune complexes, which are formed in excess in diseases of the connective tissue, and in so-called autoimmune diseases. An example of such diseases is, for example, systemic lupus erythematosus, rheumatoid arthritis, systemic vasculitis. It is the latter group of diseases that is most characterized by impaired hemostasis and a tendency to thrombosis due to such “deception” of platelets.
In addition, antiphospholipid antibodies or various toxins can be an activator of coagulation processes. Both the extrinsic pathway and the intrinsic pathway start out differently, but then they both come together to form a common stream of reactions that are catalyzed by coagulation factors V and VIII. The result in both cases will be the transformation of prothrombin into thrombin, and the polymerization of fibrinogen into fibrin.
We should not forget that platelets, which are located near the endothelium, or the inner lining of blood vessels, can not only begin to be activated when damaged, or when the vascular wall is ruptured. In some cases, the vascular endothelium may be intact, but it is significantly altered. This can occur during inflammation, for example, with the same systemic vasculitis. In this case, a normal vessel in which blood flowed without any problems turns into a site of progressive thrombus formation. Despite the fact that there is a large supply of coagulation factors in the blood, this supply can gradually become depleted. But these questions go far beyond the scope of our topic related to the review of the coagulation system.
Blood clotting phases
To completely stop bleeding, three successive phases must pass.
The first phase is the longest. The largest number of processes occurs at this stage.
To begin this phase, an active prothrombinase complex must be formed, which, in turn, makes prothrombin active. Two types of this substance are formed: blood and tissue prothrombinase.
For the formation of the first, activation of the Hageman factor is necessary, which occurs due to contact with the fibers of the damaged vascular wall. Also, high molecular weight kininogen and kallikrein are required for the functioning of factor XII. They are not included in the main classification of blood coagulation factors, however, in some sources they are allowed to be designated by the numbers XV and XIV, respectively. Next, the Hageman factor brings the Rosenthal factor XI into an active state. This leads to the activation of factors IX first, and then VIII. Antihemophilic globulin A is necessary for factor X to become active, after which it binds to calcium ions and factor V. Thus, blood prothrombinase is synthesized. All these reactions occur on the matrix of platelet thromboplastin (PF-3). This process is longer, lasting up to 10 minutes.
The formation of tissue prothrombinase occurs more quickly and easily. First, tissue thromboplastin is activated, which appears in the blood after damage to the vascular wall. It combines with factor VII and calcium ions, thus activating Stewart-Prower factor X. The latter, in turn, interacts with tissue phospholipids and proaccelerin, which leads to the production of tissue prothrombinase. This mechanism occurs much faster - up to 10 seconds.
Hemostasis
1. Concept of the blood coagulation system
2. Cellular blood clotting factors.
3. Platelet-vascular mechanism.
4. Coagulation mechanism.
5. Mechanisms and factors for maintaining blood in a liquid state.
6. Laboratory indicators of blood coagulation.
Hemostasis (blood coagulation system) is a complex system of homeostasis , which, on the one hand, maintains blood in a liquid state, ensuring normal blood supply to organs and tissues, and on the other hand, stops bleeding and prevents blood loss from the body by maintaining the structural integrity of the walls blood vessels and rapid thrombus formation when they are damaged. The significance of this system is that it prevents blood loss from the circulatory system and thereby ensures normal blood supply to organs and tissues.
Functional hemostasis system:
— Regulatory apparatus: medulla oblongata, hypothalamus, cerebral cortex;
— Working organs of the system: lungs: fibrinolysin, antithromboplastin; mast cells: antithrombin; kidneys: fibrinolysin; liver: procoagulants;
— Connecting link: XII factor, parametabolites = inflammation;
Hemostasis is realized by 3 interacting structural components:
1. The walls of blood vessels (primarily their intima (tunica intima)),
2. Blood cells, 3 plasma enzyme systems (coagulation, fibrinolytic (plasmin), kallikreininin, etc.).
This entire system is subject to neurohumoral regulation. Positive and negative feedback mechanisms clearly function in it, and therefore the blood clot that normally forms then quickly dissolves. The inner lining of blood vessels and platelets interact especially closely with each other and therefore they are combined into a common mechanism - platelet-vascular . It can also be called primary , because It is microvessels (up to 100 microns in diameter) and platelets that play the leading role in stopping bleeding in the microvasculature. The formation of fibrin ( coagulation ) clots occurs somewhat later, providing greater density and better fixation of blood clots.
We will now analyze them in this order.
Factors providing platelet-vascular hemostasis:
The following functions of platelets are especially important for hemostasis:
1. Angiotrophic - the ability to maintain the normal structure and function of microvessels, their resistance to damaging influences, impermeability to red blood cells;
2. The ability to maintain spasm of damaged vessels by secretion (release) of vasoactive substances - adrenaline, norepinephrine, serotonin;
3. The ability to clog damaged vessels by forming a primary platelet plug ( thrombus ) - a process that depends on the ability of platelets to stick to the subendothelium ( adhesion ), the ability to stick together and form lumps of swollen platelets ( aggregation ),
4. Form, accumulate and secrete, upon activation, substances that stimulate adhesion and aggregation and blood coagulation.
Platelet coagulation factors - the most important of them is membrane phospholipid factor 3 , which serves as a matrix for the interaction of plasma hemocoagulation factors and the formation of their active complexes. In its properties, this component is identical to cephalin and the membrane factor of erythrocytes - erythrocytin .
Also important is the 6th platelet factor - retractozyme , which is necessary for contraction and compaction of the fibrin clot. Platelets contain activators of the polymerization of fibrin monomers, factor V, and many plasma coagulation and fibrinolysis factors are concentrated on the surface and in their channels, and therefore their high concentration is created in the hemostatic plug (prothrombin, thromboplastin, Ac-globulin, convertin, factors II, III , V, VIII, IX, X, XI, XII, plasminogen, etc. Therefore, platelets most significantly influence the intensity and speed of local coagulation in the area of thrombus formation, and not the process of blood coagulation in general.
Red blood cell factors that promote blood clotting:
1. Thromboplastic (erythrocytin is a thermostable phospholipid corresponding to platelet factor 3).
2. Antiheparin factor.
3. Ac-globulin.
4. A factor that promotes the transition of fibrinogen to fibrin.
5. Fibrin stabilizing factor.
6. Erythrocytes enhance platelet adhesion and aggregation by releasing ADP.
7. Red blood cells adsorb heparin and heparin-like substances, delivering them to the kidneys.
8. Red blood cells take up urokinase and reduce fibrinolysis.
However, red blood cells contain factors that prevent blood clotting:
1. Antithromboplastic factor.
2. Antithrombins.
3. Red blood cells contain substances that help dissolve a blood clot - for example, erythrokinase . If the formation of a blood clot is accompanied by hemolysis, then the released proactivator and plasminogen activator contribute to rapid blood thinning. Thus, destroyed red blood cells mainly stimulate fibrinolysis , but intact ones prevent it. Those. Under natural conditions, the inclusion of red blood cells in the blood clot makes it more resistant to plasmin.
Leukocytes contain thromboplastin factor, reminiscent of platelet factor 3, antiheparin factor, AGG, XII. Leukocytes are able to aggregate and adhere to the wound surface, especially under the influence of ADP, enhancing the formation of a fibrin clot at the beginning of the injury. In a number of pathological conditions, a procoagulant ( thromboplastin ) is released from leukocytes and hypercoagulation develops - disseminated intravascular coagulation.
On the other hand, in later stages of injury, they prevent platelet adhesion, promoting blood movement and nutrition to the injured areas. Basophils contain heparin, and neutrophils contain an antithrombin and antithromboplastic anticoagulant. Granulocytes maintain the blood in a fluid state by secreting heparin, plasminogen, proactivator and activators of fibrinolysis.
Vascular wall factors that promote blood clotting:
1. Thromboplastin - extracts from the inner layer of the vessel have the greatest thromboplastic effect, and capillary epithelial cells have the least. This is of great biological significance, because For hemostasis in capillaries with slow blood flow, the internal hemostasis system should be of greater importance. In large arteries, trauma activates the extrinsic coagulation mechanism and stops bleeding. With atherosclerosis and with age, the content of thromboplastin in the vessel wall decreases.
2. In the wall of the vessel there is an antiheparin factor - a compound that binds heparin and thereby accelerates blood clotting. The factor is found in all layers of the aorta, coronary arteries, portal and vena cava, and various tissues of the heart. With age, its concentration changes slightly and its role, apparently, is to neutralize anticoagulants in tissue damage.
3. There is a compound resembling convertin in the vessels, and it is likely involved in the formation of prothrombinase.
4. Various tissues (brain, kidneys, lungs, muscles, etc.) contain a fibrin-stabilizing factor. It is also present in blood vessels.
The vascular endothelium has high thromboresistance and plays an important role in maintaining the liquid state of circulating blood due to its following features:
1. The endothelium is capable of forming and releasing into the blood a powerful inhibitor of platelet aggregation - prostacyclin.
2. The endothelium produces tissue fibrinolysin activator.
3. The endothelium is not capable of contact activation of the holy blood system.
4. The endothelium creates an anticoagulant potential at the blood/tissue interface by fixing the heparin-antithrombin-III complex.
5. The endothelium is capable of removing activated blood clotting factors from the bloodstream.
The walls of blood vessels are able to withstand not only blood pressure, but also moderate external traumatic influences, preventing the development of hemorrhages. This depends both on the usefulness of the endothelium and on the structural features of the subendothelial layer - the degree of development and quality of collagen and microfibrils, the ratio of collagen and elastic fibers, the structure of the basement membrane, etc.
In many ways, these properties depend on platelets - their quantity and qualitative characteristics, and therefore, with insufficient platelet content or their qualitative defects, both diapedetic bleeding (in the absence of mechanical damage to the vessels) and the fragility of microvessels increase: petechiae and bruises easily occur, tests for fragility of capillaries (eg pinch, cuff, etc.).
When vessels are damaged and the subendothelium is exposed, hemostasis is activated in various ways:
1. Release into the blood of tissue thromboplastin (factor III, apoprotein III) and other coagulation activators, as well as platelet stimulants - adrenaline, norepinephrine, ADP,
2. Contact activation by collagen and other components of the platelet subendothelium (adhesion) and blood coagulation (activation of factor XII),
3. Production of plasma cofactors of platelet adhesion and aggregation (von Willebrand factor, etc.).
Thus, the vascular wall interacts most closely with all parts of hemostasis, especially with platelets.
Platelet-vascular hemostasis.
The main role in the implementation of primary hemostasis belongs to platelets. Due to damage to blood vessels, platelets come into contact with the subendothelium - mainly with the main adhesion stimulator - collagen - they swell, form processes and stick. The duration of this phase is 1-3 seconds. This requires Ca ions and a protein synthesized in the endothelium - von Willebrand factor (VIII, VWF), and in platelets - membrane glycoprotein Ib (GP-Ib) interacting with this factor, which in its absence leads to Bernard-Soulier disease.
Following adhesion, rapid aggregation of platelets occurs at the site of damage - phase II (tens of seconds), which leads to rapid growth of the thrombus. The primary stimulus for aggregation is provided by collagen and, to an even greater extent, by ADP, catecholamines and serotonin, released from the vascular wall, from platelets hemolyzing in the area of damage and platelets that have already adhered.
second are actively secreted : adrenaline, norepinephrine, serotonin, antiheparin factor 4. Later, granules containing lysosomal enzymes are secreted.
As a result of the interaction of platelet and plasma factors in the hemostasis zone, thrombin is formed, small doses of which sharply enhance and complete the aggregation process and at the same time trigger blood clotting, as a result of which the platelet clot acquires greater density and undergoes retraction - phase III - viscous metamorphosis.
After platelet aggregation and fibrin formation, under the influence of retractozyme, a special contractile protein of platelets, thrombostenin, is reduced, which leads to the convergence of platelets and fibrin strands. Retraction requires thrombin to promote viscous metamorphosis.
In the regulation of platelet hemostasis, derivatives of arachidonic acid, released from membrane phospholipids of platelets and the vascular wall due to activation of phospholipases, play an important role. Under the influence of cyclooxygenase, prostaglandins are formed, from which an extremely powerful aggregating agent, thromboxane-A2, .
The lifespan of thromboxane, prostacyclin and other prostaglandins is several minutes, but their importance in the regulation and pathology of hemostasis is very great. This mechanism is a trigger in the development of AAFT (adhesive-aggregation function of platelets). To carry out AAPT, a number of plasma aggregation cofactors are required - calcium and magnesium ions, fibrinogen, albumin and two protein cofactors, referred to in the literature as aggrexons A and B, phospholipid cofactor, etc.
At the same time, paraproteins, cryoglobulins and fibrinolysis products inhibit platelet aggregation.
To carry out the aggregation function, platelet membrane glycoproteins that interact with aggregating agents are very important:
1) glycoprotein 1, consisting of two subunits – 1a and 1b. The first is a von Willebrand factor receptor and is necessary for adhesion, the second is for thrombin aggregation (their decrease leads to von Willebrand disease, Bernard-Soulier disease;
2) glycoprotein 2 (also of two subunits) is necessary for all types of aggregation (with its deficiency, Glanzmann’s thrombocytoastenia develops);
3) glycoprotein 3, one of whose components binds to Hb and calcium and is necessary for most types of clot aggregation and retraction.
Coagulation hemostasis.
Blood coagulation is a complex multi-stage process that involves a number of protease proteins, non-enzymatic accelerator proteins that ensure the interaction of coagulation factors on phospholipid matrices (platelet factor 3, micromembranes of other cells), and calcium ions.
It is conventionally divided into 3 phases:
1 — multistage formation of thromboplastin;
2 - thrombin formation;
3 - the final stage, where, under the influence of thrombin, fibrinogen is first converted into fibrin monomers, and then into its polymer, stabilized by activated factor XIII.
Nomenclature . According to the international nomenclature, all plasma coagulation factors are designated by Roman numerals in the order of discovery of the factors (less often - by function, names of authors or patients).
Plasma factors:
– fibrinogen – euglobin, molecular weight 400,000-500,000 Yes, is formed in all organs and tissues that have a system of phagocytic mononuclear cells (SPMN) (most in the liver), is found in plasma, lymph, BM, trans- and exudate (plasma content 200 -400 mg%, decreases with liver diseases, menstruation, increases with pregnancy, infectious diseases, inflammatory processes, in the postoperative period). It is an inactive form of the fibrin protein and is converted to fibrin under the influence of thrombin.
– prothrombin (thrombogen) – euglobin (glycoprotein), is formed in the SFMN (mostly in the liver, less in other organs) with the participation of vitamin K, passes into the active form – thrombin.
– tissue thromboplastin,
– Ca2+ ions,
– plasma Ac-globulin, proaccelerin, labile factor,
– serum Ac-globulin, accelerin, active form of factor V,
– proconvertin, a stable factor, is formed with the participation of vitamin K in the liver,
– antihemophilic globulin A (AGGA),
- plasma component of thromboplastin, antihemophilic globulin B, Christmas factor, formed with the participation of vitamin K.
– antihemophilic globulin C, Stewart-Prower factor, prothrombinase, formed with the participation of vitamin K.
- plasma thromboplastin precursor (PPT), Rosenthal factor, is formed with the participation of vitamin K.
– contact factor, Hageman factor – after activation, remains on the surface of the damaged vessel, which prevents the generalization of the blood coagulation process; activates the kallikrein system, complement system and fibrinolysis.
- fibrin-stabilizing factor (fibrinase, fibrinoligase, transglutaminase), formed in the liver.
– kallikrein.
- kininogen.
To indicate the activated factor, the letter “a” or “f” is added to these numbers if one of the fragments of the factor becomes the active principle.
According to the modern cascade-complex theory of blood coagulation, activation of prothrombin (factor II) is the result of a multi-stage enzymatic process in which various coagulation factors are sequentially activated and interact with each other. Of these, factors III, VII, IX, X, XI and XII, as well as prekallikrein are protease , and factors VIII and V are non-enzymatic accelerators of the process, accelerating the interaction and activation of enzymatic factors many thousands of times.
There are two main mechanisms for triggering the coagulation process - external and internal. In the external mechanism, blood coagulation is stimulated by the release of tissue thromboplastin (factor III or phospholipid-apoprotein III complex) into the plasma. In the internal mechanism, blood coagulation occurs without the participation of tissue thromboplastin. The triggering factor here is factor XII (Hageman), activation of which occurs either due to contact with a foreign surface (glass, metal) or due to its enzymatic cleavage by kallikrein, plasmin and other proteases, or upon contact with the subendothelium (collagen) and other components of the connective tissue. tissue for injuries, vasculitis, atherosclerosis.
Scheme of the cascade-complex mechanism of blood coagulation:
The mechanism of transformation of fibrinogen into fibrin.
The essence of this stage is that the proteolytic enzyme thrombin (formed from prothrombin) cleaves two peptides A and two peptides B from the fibrinogen molecule. As a result, fibrin monomers are formed, each of which has 4 free bonds. These bonds connect to each other, first in pairs (dimers), and then into a polymer (connecting end to end and side to side) and fibrin fibers are formed.
This fibrin is soluble (in 5-7 M urea and 2% monochloroacetic acid) and is designated fibrin S (soluble). Under the influence of factor XIIIa (which is also activated by thrombin in the presence of Ca2+ ions), additional disulfide bonds are formed in fibrin between the Y- and a-chains. Fibrin I, insoluble in urea, is formed.
Physiological anticoagulants are necessary to maintain blood in a fluid state and to limit the process of thrombus formation.
They are divided into two main groups:
1. Primary, or independently synthesized and constantly contained in the blood,
2. Secondary, formed during the process of proteolysis during blood coagulation and fibrinolysis.
Among the primary ones, the following inhibitor proteins are the most important:
Heparin is a natural anticoagulant (together with fibrinolysin, it is part of the physiological anticoagulant system of the blood). Produced in basophils and mast cells. Heparin directly affects blood clotting factors, blocking or reducing their activity. When administered intravenously, the effect occurs almost instantly and lasts 4-6 hours. Heparin is destroyed in tissues with the participation of heparinase (uroheparin is formed, which is excreted through the kidneys). Heparin has antithromboplastin, antiprothrombin and antithrombin effects, delays the transition of fibrinogen to fibrin, increases fibrinolysis, in large doses inhibits platelet aggregation and adhesion, increases vascular permeability.
Antithrombin III is a universal inhibitor of almost all enzymatic coagulation factors, primarily thrombin IIa and Xa. It accounts for more than 75% of the total anticoagulant activity of plasma. It is the main plasma cofactor of heparin, and if there is little antithrombin III in the blood, then there is no point in administering heparin to the patient to treat thrombosis. With a hereditary or acquired decrease in antithrombin III, a severe thrombophilic state occurs with recurrent thrombosis of the main veins of the extremities and internal organs, pulmonary embolism, and organ infarctions.
Secondary physiological anticoagulants are formed during the process of blood coagulation and fibrinolysis as a result of further enzymatic degradation of a number of coagulation factors. After initial activation, they lose their ability to participate in hemostasis and often acquire anticoagulant properties. Thus, fibrin adsorbs and inactivates large amounts of thrombin (and is designated antithrombin I ). The products of the enzymatic breakdown of fibrinogen/fibrin by plasmin (fibrinolysin) inhibit both platelet aggregation and the self-assembly of fibrin monomers - i.e. fibrin formation. Adrenaline, in combination with fibrinogen and heparin, is transformed from a stimulator of platelet aggregation and blood coagulation into a factor that prevents hemocoagulation and into an activator of non-enzymatic fibrinolysis.
Fibrinolysis - an enzyme system (causing asymmetric cleavage of fibrin/fibrinogen into smaller and smaller fragments) is called fibrinolytic or plasmin . The main component of this system is the enzyme plasmin (fibrinolysin), contained in plasma in the form of a proenzyme - plasminogen. Active plasmin is quickly blocked by antiplasminogens and removed from the bloodstream. When streptokinase or urokinase is administered, the level of plasminogen in the blood decreases very quickly and deeply due to the transition to active plasmin, and then recovers within 18-28 hours. In the body, activation of fibrinolysis (as well as activation of coagulation) can be either external or internal .
Internal activation of fibrinolysis (as well as coagulation) is due to the complex XIIa or XIIf with kallikrein (XIV) and high molecular weight kininogen (XV).
External activation is carried out mainly by a tissue-type protein activator synthesized in the vascular endothelium. Its intense release occurs with all types of vascular blockage or compression, under the influence of vasoactive substances and medications.
Mechanisms and factors for maintaining blood in a liquid state.
Maintaining blood in a liquid state is carried out due to the presence of anticoagulants, the activity of which should be higher than that of coagulants. Taking into account the many coagulation factors, there is a powerful anticoagulant . It contains antithromboplastins, antithrombins, and enzymes that prevent the transition of fibrinogen to fibrin. When thrombin the blood, it irritates the chemoreceptors of the vascular wall. From here, irritation is reflexively transmitted to the medulla oblongata and, as a result, heparin and heparin-like anticoagulants are released from the vascular wall, which delay the formation of fibrin and its conversion into fibrinogen (spherical shape). For the discovery of this mechanism, Academician B.A. Kudryashov received the Lenin Prize.
Laboratory values to determine the risk of bleeding or thrombosis:
I. Integral indicators that give a summary idea of the coagulation system.
a) general indicators of platelet-vascular hemostasis,
1. Mechanical stability of capillaries (pinch test, number of petechiae),
2. Duration of bleeding from a puncture of a finger or earlobe,
3. Indicators of platelet aggregation.
b) indicators of coagulation hemostasis: blood clotting time (4-8 minutes), changes with severe disorders: lengthens with hemophilia, shortens with thrombosis.
II. Samples characterizing individual stages of coagulation or platelet-vascular hemostasis.
Determination of individual phases of hemostasis:
Phase I:
a) plasma recalcification time is 1.5 - 2 minutes, based on it a
b) autocoagulation test - a hemolysate is prepared from the patient’s blood in a hypotonic Ca2+ solution and its thromboplastic activity in relation to the patient’s or donor’s plasma is determined, and the dynamics of plasma coagulation are studied.
c) plasma tolerance to heparin.
d) thromboplastin test, showing the activity of thromboplastin and if the autocoagulation test determined normal parameters of phases II and III of coagulation, then changes in the test will be associated only with phase I pathology.
Phase II:
a) prothrombin test - tissue (already active) thromboplastin is added to citrated plasma, which activates factors II and I. The patient's prothrombin time is determined (normally 13-15 seconds). the prothrombin index is determined while simultaneously determining the prothrombin time of a healthy person · 100 =%. The norm is 80-100%, less prothrombin time of the patient means hypocoagulation, more means hypercoagulation. And since factors V, VII, VIII and X are synthesized in the liver, the test characterizes its protein-forming function.
b) thrombin test - when thrombin is added to plasma, an increase in time indicates an increase in anticoagulants or a decrease in fibrinogen.
III phase:
a) determination of the amount of fibrinogen,
b) determination of thrombotest.
III. Activity of individual coagulation factors or anticoagulants.
1: determination of anticoagulants:
a) antithrombin activity according to thrombin test 20-32 sec,
b) determination of free heparin by acceleration of thrombin time after binding of heparin and similar anticoagulants with protamine sulfate for 5-10 seconds,
c) determination of fibrinolytic activity of plasma by the dissolution time of a standard fibrin clot: 10-25% dissolves in an hour,
2: a) determination of platelet count 200-400·109/l,
b) determination of the blood clot retraction index (0.3-0.5/hour).
c) the rate of adhesion is determined by how many platelets remain in suspension after contact with glass beads or glass - decreased adhesion - tendency to bleeding, increased - to thrombosis,
d) platelet aggregation: spontaneous (natural) and induced . Determined by changes in the optical density of platelet suspension. The higher the aggregation, the more the optical density changes. The norm is 18-20%.
Second and third phases
The second phase begins with the conversion of prothrombin into active thrombin through the functioning of prothrombinase. This stage requires the action of plasma coagulation factors such as IV, V, X. The stage ends with the formation of thrombin and occurs in a few seconds.
The third phase is the conversion of fibrinogen into insoluble fibrin. First, fibrin monomer is formed, which is ensured by the action of thrombin. Next, it turns into fibrin polymer, which is already an insoluble compound. This occurs under the influence of a fibrin-stabilizing factor. After the formation of a fibrin clot, blood cells are deposited on it, which leads to the formation of a blood clot.
Then, under the influence of calcium ions and thrombostenin (a protein synthesized by platelets), clot retraction occurs. During retraction, the clot loses up to half its original size as blood serum (plasma without fibrinogen) is squeezed out. This process takes several hours.
What does the platelet count mean in a blood test?
Platelets are small blood cells 2-4 microns in size. Their name comes from the Greek words for “clot” and “cell,” which pretty well describes their ability to form blood clots. These blood elements have a spherical shape, do not have a nucleus and color, and are formed in the cells of the red bone marrow. The main function of platelets is to maintain the functioning of blood vessels. In the human body, platelets are “responsible” for the following processes:
- The primary (physical) closure of blood vessels at the sites of their damage to prevent blood loss is an outpost and the first line of defense organized by platelets in preserving the liquid state of blood within the veins, arteries, and capillaries. This is achieved through numerous interactions between the components of the vessel wall, blood cells and plasma clotting factors contained in the outer layer of the platelet.
- Plasma coagulation (formation of a permanent platelet plug) begins as a result of the release of phospholipids (glycoproteins) from the platelet membrane and a-granules with 22 active components: high molecular weight proteins, calcium ions and low molecular weight organic substances - they trigger the process of blood clotting, being its factors. Along with internal platelet clotting factors, 12 plasma factors (contained in blood plasma) are also known. All of them are interconnected and form a complex sequential chain, a cascade of biochemical reactions, leading to the formation of a dense fibrin clot, its compaction, and after healing of the wound, its resorption.
- Regeneration and angiotrophic function. By donating part of its surface to “patches,” the platelet provides nutrition to the cells of the vascular wall (endotheliocytes), maintaining the structure and functions of microcapillaries. This occurs due to the absorption of platelets by endothelial cells. When destroyed, the platelet releases other “factors” - growth factors: VEGF, EGF, PDGF, TGF-β, FGF and others, which are responsible for cell repair and division. Every day, the endothelium (the inner protective layer of blood vessels, consisting of endothelial cells) absorbs approximately 35 g/l of platelets.
Fibrinolysis
To prevent the resulting blood clot from completely blocking the lumen of the vessel and stopping the blood supply to the corresponding tissues, there is a fibrinolysis system. It ensures the breakdown of the fibrin clot. This process occurs at the same time as the thickening of the blood clot, but is much slower.
To carry out fibrinolysis, the action of a special substance - plasmin - is necessary. It is formed in the blood from plasminogen, which is activated due to the presence of plasminogen activators. One such substance is urokinase. Initially, it is also in an inactive state, beginning to function under the influence of adrenaline (a hormone secreted by the adrenal glands) and lysokinases.
Plasmin decomposes fibrin into polypeptides, which leads to the dissolution of the blood clot. If the mechanisms of fibrinolysis are disrupted for any reason, the blood clot is replaced by connective tissue. It can suddenly break away from the vessel wall and cause a blockage somewhere else in the organ, which is called a thromboembolism.
Diagnosis of hemostasis
If a person has a syndrome of increased bleeding (heavy bleeding during surgical interventions, nosebleeds, uterine bleeding, causeless bruising), it is worth suspecting a blood clotting pathology. To identify the cause of coagulation disorders, it is advisable to take a general blood test and a coagulogram, which will display the state of coagulation hemostasis.
It is also advisable to determine coagulation factors, namely factors VIII and IX. Since a decrease in the concentration of these compounds most often leads to blood clotting disorders.
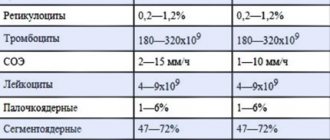
The main indicators characterizing the state of the blood coagulation system are:
What tests can help?
Finally, in conclusion, we should very briefly describe what research methods can assess the potential of the internal and external pathways of activation of the coagulation system. Of the simplest coagulation tests, the internal activation pathway can be determined through APTT (activated partial thromboplastin time). The cost of analysis in modern private laboratories is approximately 175 rubles.
Normally, for a patient, this time ranges from 25 to 37 seconds. And an increase in the duration of blood clotting, or a slowdown in time, speaks of various types of hemophilia, diseases of the corresponding factors affecting blood clotting - Willebrandt or Hageman. The time is also prolonged when treated with heparin, as well as in the presence of antiphospholipid syndrome.
The second fairly simple “coagulation” analysis is a study of prothrombin time and INR (international normalized ratio). The cost of this test in the laboratory is about 270 rubles, and this test is a coagulation test that shows how the patient's plasma coagulates after adding a special mixture of thromboplastin and calcium to it as a seed. Normally, prothrombin time ranges from 78 to 142%. The second name of this study is the prothrombin index, and there is a separate article about this. If it is higher than these values, then the patient has a high tendency to thrombosis, and if it is lowered, then this may indicate a poorly functioning liver and vitamin K deficiency.
Pathology of hemostasis
The most common disease observed with clotting factor deficiency is hemophilia. This is a hereditary pathology transmitted along with the X chromosome. Mostly boys are affected, but girls can be carriers of the disease. This means that girls do not develop symptoms of the disease, but they can pass the hemophilia gene to their offspring.
With a deficiency of coagulation factor VIII, hemophilia A develops; with a decrease in the amount of factor IX, hemophilia B develops. The first option is more severe and has a less favorable prognosis.
Clinically, hemophilia is manifested by increased blood loss after surgical interventions, cosmetic procedures, and frequent nasal or uterine (in girls) bleeding. A characteristic feature of this pathology of hemostasis is the accumulation of blood in the joints (hemarthrosis), which is manifested by their pain, swelling and redness.
Diagnosis and treatment of hemophilia
Diagnosis consists of determining the activity of factors (significantly reduced), conducting a coagulogram (extending the clotting time and APTT, increasing the time of plasma recalcification).
Treatment of hemophilia consists of lifelong replacement therapy with clotting factors (VIII and IX). Drugs that strengthen the vascular wall (Trental) are also recommended.
Thus, blood clotting factors play an important role in ensuring the normal functioning of the body. Their activity ensures the coordinated functioning of all internal organs by supplying them with oxygen and essential nutrients.