Oncology
12/18/201701/24/2019 Chernenko A. L. 2219 Views cyfra 21 1, oncology
The problem of morbidity and high mortality from cancer is that it begins to manifest clinically in late stages, when the malignant tumor metastasizes, and the prognosis is most often unfavorable. An as yet undetectable disease can be detected using a special analysis for tumor markers. These compounds usually appear in the blood as the body's response to the appearance of cancer cells or are the result of the vital activity of the tumor itself.
Since there are no or very few of them in a healthy body, an increase in the level can indicate the presence of a tumor process. About 20 antigens are diagnostically significant, one of which is the tumor marker Cyfra 21.1 (a fragment of cytokeratin 19).
- 1 What does Cyfra 21 1 mean in a blood test?
- 2 Cyfra 21 1 tumor marker – interpretation and norm
- 3 Indications for testing
- 4 How to prepare for a blood test?
Basic information
Cytokeratins are elements that form the framework of epithelial cells (cytoskeleton). Normally, they do not enter the blood and are only detected during laboratory testing when the epithelium is destroyed. There are 25 types of cytokeratins in total. Cyfra 21-1 is a fragment of the 19th, which is detected in certain types of cancer (large cell, squamous cell, etc.).
An increase in the concentration of Cyfra 21-1 in the blood serum is most characteristic of non-small cell lung cancer. But since lung tumors are very diverse in their histological structure, experts recommend conducting a comprehensive examination, including at least 3 types of tumor markers:
- CEA (carcinoembryonic antigen),
- NSE (neuron specific enolase)
- and Cyfra 21-1.
The table below shows the most acceptable examination regimens for various types of tumors
| Histological structure of the tumor | Main marker | Test sensitivity | |
| Small cell carcinoma | Cyfra 21-1 NSE | 52,3% | |
| Non-small cell carcinoma | Adenocarcinoma | Cyfra 21-1 REA | 63,2% |
| Squamous | Cyfra 21-1 | 72 – 75% | |
| Large cell | Cyfra 21-1 REA | 72 – 75% | |
| Unknown | Cyfra 21-1 NSE REA | 62 – 65% | |
The concentration of Cyfra 21-1 increases in proportion to the stage of the malignant process, which is actively used to predict the outcome of the disease and patient survival. Accordingly, a moderate increase in Cyfra 21-1 values may indicate both a progressive (slowly increasing) course of the pathology and different types of tumor.
If the tumor marker level was elevated at the time of diagnosis, then this information can be used as a “starting point” in the patient’s management program in the future. A decrease in Cyfra 21-1 values after surgical treatment reflects the degree of success of the surgical intervention and the presence/absence of residual cancer tissue.
Suspect the persistence of tumor remnants if the rate of decline of Cyfra 21-1 is too slow or the values never reach reference values. Against this background, the again increasing concentration of the tumor marker indicates a relapse of the disease. But the most important thing is that this sign is detected long (more than 12 months) before the actual growth of the tumor itself, i.e. when clinical symptoms of cancer recurrence have not yet appeared. And this can serve as a serious reason for referral for early X-ray examination.
Equally important is the Cyfra 21-1 test for monitoring invasive bladder cancer if the tumor marker concentration was elevated at the time of diagnosis. Together with CA 19-9 and carcinoembryonic antigen (CEA) testing, the CYFRA 21-1 test can be used to diagnose, assess prognosis and monitor treatment of colon cancer.
Determining Cyfra 21-1 levels in patients with severe chest trauma can predict the development of acute respiratory distress syndrome and pneumonia and prevent these complications in a timely manner.
Nuances of preparation
The Cyfra 21-1 tumor marker is quite sensitive to various factors, so before donating blood for testing, preliminary preparation will be required:
- 3-5 days before donating blood, it is important to normalize your diet by eliminating fatty and fried foods, smoked foods, and sweet confectionery. The menu consists of fresh vegetables and fruits, lean meats, dairy and fermented milk products.
- Complete abstinence from alcohol and low-alcohol drinks for the entire period of diagnosis.
- Stop using any medications. If the patient is forced to take medications on an ongoing basis, the laboratory technician is informed about this in advance.
- Donate blood on an empty stomach in the first hours after waking up, since it is during this period of time that the most reliable results can be obtained.
- Limit physical activity the day before donating blood.
- Avoid stress and emotional turmoil.
- Have a good rest and sleep the night before.
Failure to follow these recommendations may cause false results, which will significantly complicate further diagnosis.
Indications
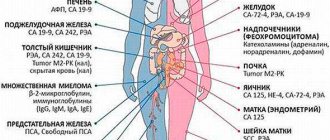
- Primary diagnosis of lung cancer (comprehensive examination);
- Assessing the effectiveness of lung cancer treatment;
- Prediction of non-small cell lung cancer outcome and patient survival;
- Monitoring for invasive bladder cancer;
- Suspicion of cancer of a different form and location;
- Diagnosis, assessment of prognosis and control of treatment of colon and breast cancer;
- Determination of treatment tactics for metastatic differentiated thyroid cancer;
- For symptoms of lung cancer (cough, hemoptysis, shortness of breath, signs of tumor compression of surrounding structures (hoarseness, difficulty swallowing).
- For symptoms of colon cancer: anemia, rectal bleeding, abdominal pain, constipation or diarrhea;
- Preoperative assessment of the Cyfra 21-1 level in a malignant thymic tumor allows one to assess the stage of the process, tumor histotype and predict relapse;
- The Cyfra 21-1 assay is used to detect recurrent cervical cancer.
The results of the analysis on Cyfra 21-1 are interpreted by specialists:
- oncologist,
- pulmonologist,
- endocrinologist,
- gastroenterologist,
- surgeon.
Content:
- Purpose of blood test
- Reasons for increased tumor markers in the blood
- Disadvantages of the method
- Cyfra 21-1 analysis - pros
← Ovarian tumor markers HE-4 and CA-125 using the Roma method
Blood test for tumor marker S-100 of skin melanoma →
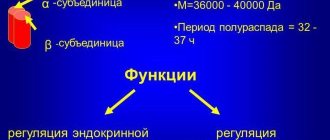
There are more than 25 groups of soluble scaffold proteins—cytokeratins. Their molecular weight is 40-68 kDa. From inside the epithelial cell, they are busy forming a framework - the cytoskeleton. It maintains mechanical cellular stability and protects against damage. The classification includes cytokeratins. They consist of 2 groups: type I - acidic, type II - basic. Single-row epithelium contains cytokeratins with low molecular weight, stratified epithelium - keratinizing and non-keratinizing - with high mass.
Immunohistochemical studies have proven that the synthesis of cytokeratins 7, 8, 18 and 19 occurs in the epithelium of the respiratory tract. As soon as cytokeratin enters the blood, including cyfra 21-1, cell destruction and necrosis of tumor masses occurs.
We also recommend studying this topic:
Tumor marker HE4 is a marker of the pelvic organs in women
Cyfra boost 21-1
Important! The interpretation of the results is always carried out comprehensively. It is impossible to make an accurate diagnosis based on only one analysis.
- Lung cancer;
- Bladder cancer;
- Other tumor locations (cervix, esophagus, thyroid gland, head and neck, colon);
- Recurrence of cervical cancer;
- Papillary thyroid carcinoma;
- Metastatic cancer (distant metastasis of a tumor, for example, the breast to other organs and tissues).
- colon adenocarcinoma;
- Breast cancer metastases, sensitivity 65% (together with tests for CEA and CA 15-3, sensitivity is about 90%).
Decoding and norms of results
After the study has been carried out, the data is recorded on a special form. Next to the available values, the normal values are usually indicated, as well as the reasons for the deviations.
Different laboratories have their own norms, which depend on many factors. Therefore, before making a conclusion, you should compare the available data with the standards established by the laboratory itself.
The average normal values for the Cyfra 21-1 tumor marker are 0-3.3 ng/ml. Values exceeding the norm indicate a high probability of developing an oncological process in the patient’s body.
If the question remains open regarding the results obtained, the patient may be scheduled for a repeat study in 2-3 weeks. It should be carried out in the same laboratory, which will allow the dynamics to be assessed.
The absence of a positive result when the test is repeated indicates the absence of cancer. A single slight excess of the norm may be associated with the progression of other diseases not related to oncology.
When is a CK19 soluble fragments test prescribed?
An analysis of this substance is prescribed by a specialist in cases where the following signs are present:
- Shortness of breath, problems with swallowing, a shrunken voice, a lingering cough, first dry, then with bloody sputum. All of these symptoms may indicate a lung tumor.
- PNS (paraneoplastic syndrome) - increased calcium in the blood plasma, Cushing's syndrome and myasthenic syndrome.
- Signs of colon cancer include anemia, rectal bleeding, diarrhea or constipation, pain in the stomach area.
- If there is a suspicion of a tumor of the glandular tissue of the breast.
- When prescribing therapy for metastatic thyroid cancer.
Reasons for increasing or decreasing tumor markers
There are several prerequisites that can provoke a change in the level of these specific substances:
- a newly detected increase, especially if a person has negative symptoms, indicates the development of some pathological process in his body;
- if the level of biomolecules began to decrease after therapeutic measures, surgery, chemotherapy or radiation, this indicates their effectiveness and the onset of remission.
Important! If, when conducting this test to monitor the treatment course, its results reveal a sharp increase in the amount of biomolecules that has decreased almost to normal, experts say that the tumor marker shows throat cancer that has entered the relapse stage.
Indications for testing for tumor markers of throat cancer
Regular diagnosis of throat and larynx cancer through blood tests to determine the level of tumor markers is not necessary for all people. This oncotest should be taken annually only by those whose family history is burdened with a predisposition to oncology (a dangerous disease was identified in blood relatives) and patients who are registered for benign and obligatory tumors, i.e., necessarily subject to malignancy over time, precancerous diseases. Such pathologies include hyperplastic laryngitis, papillomas, cysts, fibromas, and oral leukoplakia.
In addition, there may be a need to take tumor markers for throat cancer on an emergency basis.
It is due to the following prerequisites:
- unexpected appearance in the laryngeal area of hyperplasia, plaques and compactions of unknown origin;
- exacerbation of long-term and often recurrent infectious and inflammatory processes of the upper respiratory tract.
It is mandatory to study tumor markers after antitumor therapy. Using the results obtained, the doctor can assess the effectiveness of the prescribed treatment course, as well as the dynamics of the development of the pathological process and promptly make adjustments to therapeutic prescriptions until positive dynamics are achieved. A test is performed to determine markers of squamous cell carcinoma and if distant metastases are suspected.
Important! Experts recommend regularly taking tumor markers for cancer of the larynx and throat for people who have a long history of smoking, exceeding 10 years, or who have worked in hazardous work for a long time, since they are in the main risk group for the appearance of cancer in the laryngeal area. In addition, this test is recommended for those who have carious teeth and do not visit the dentist often enough, since caries can provoke the appearance of infectious and inflammatory foci in the laryngeal area, which can become malignant.
When does it go down?
CYFRA 21-1 may be lowered due to certain metabolic characteristics or dietary preferences. False-negative results are often observed in women during the menopausal period, during early and late pregnancy, or while breastfeeding. It requires mandatory testing over time, because the lack of further diagnosis can lead to advanced stages of the disease. The indicator of this study is often reduced in the following conditions:
If the oncological marker is not determined, then the treatment used is effective and the cancer recedes.
- the level of the substance in the blood has not yet increased to the level of cancer detection;
- tumor tissue does not synthesize such a tumor marker;
- effect of treatment;
- no cancer.
When is the indicator increased?
With high levels of this substance in the blood, conclusions are drawn regarding the following diagnoses:
- Small cell carcinoma of lung tissue. With it, the marker is especially sharply increased.
- Bladder carcinomatosis. High concentrations of the substance are found in muscle-invasive types of tumors.
- Benign neoplasms localized in the lungs, liver and bladder.
- Chronic renal failure. With it, creatinine and other “toxins” will also increase.
- Smoking abuse. This bad habit often leads to false positive results.
- Benign tumors in the kidneys or abdominal organs. These include hepatocellular hemangioma or fibroadenomas.
How long does it take to analyze and are there possible misfires?
The results of a test that examines tumor markers for throat cancer are usually ready within 1 business day, but it should be said that it cannot give a 100% guarantee as to whether malignant cells are present in the larynx of a particular person or not. There are factors in which the tumor marker SCC or Cyfra21-1 can show false-positive results even in completely healthy people, and false-negative results in patients. These include stress, physical fatigue and much more. That is why you should not diagnose yourself based on the results you see.