© Author: Z. Nelly Vladimirovna, laboratory diagnostics doctor at the Research Institute of Transfusiology and Medical Biotechnology, especially for SosudInfo.ru (about the authors)
Iron (ferrum, Fe) is one of the most important elements for the body. Almost all the iron that comes from food is bound to proteins and subsequently becomes part of them. Everyone knows such an iron-containing protein as hemoglobin, which consists of a non-protein part - heme and globin protein. But in the body there are proteins that contain iron, but do not have a heme group, for example, ferritin, which provides a reserve of the element, or transferrin, which transports it to its destination. An indicator of the functionality of the latter is total transferrin or total iron binding capacity of serum (TIBC) - this analysis will be discussed in this work.
Transport protein (transferrin - TF, Tf) in the body of healthy people cannot “ride empty”, that is, saturation with iron should not be less than 25 - 30%.
The normal level of THC is 40.6 – 62.5 µmol/l. The reader can find more detailed information regarding normal values in the table below, however, as always, it should be borne in mind that standards may differ from one source to another and from one laboratory to another.
Carries as much as he can take
Typically (if everything in the body is normal) approximately 35% of the transport protein is associated with Fe. This means that this protein takes up for transfer and subsequently transports 30–40% of the total amount of the element, which corresponds to the same percentage (up to 40%) of transferrin binding capacity (iron binding capacity of serum - IBC).
In other words: TIBC (total iron-binding capacity of serum) in laboratory work is an analysis that indicates not the concentration of the transport protein, but the amount of iron that can be “loaded” onto transferrin and sent to the bone marrow for erythropoiesis (formation of red blood cells) or to places where the item's inventory is stored. Or it can (also, being associated with tronferrin) go in the opposite direction: from “storages” or from sites of decay (phagocytic macrophages).
In general, iron moves throughout the body and gets where it needs to thanks to the protein transferrin, which is a kind of transport vehicle for this element.
General information
Latent (unsaturated) iron binding capacity of serum (LZhSS, UIBC, UIBC) is an indicator used to identify iron deficiency in the body.
The main indications for use: differential diagnosis of anemia, liver diseases (acute hepatitis, cirrhosis), nephritis, evaluation of treatment with iron supplements, various chronic diseases, pathology of the gastrointestinal tract and associated impaired absorption of iron. Normally, transferrin is saturated with iron by approximately 30%, and the additional amount of iron that can bind to transferrin is called the latent (unsaturated) iron-binding capacity of the serum. LVCC or NIBC is the difference between the total iron-binding capacity (TIBC) and the actual saturation of transferrin. This is expressed by the formula: LZhSS(NZhSS) = OZhSS - Serum iron.
Total iron binding capacity of serum (TOIBC, Total Iron Binding Capacity, TIBC) is the maximum amount of iron that can bind transferrin until complete saturation. It is established as the sum of indicators - Serum iron + latent (unsaturated) iron binding capacity of serum (LZhSS, UIBC - from the English. Unsaturated Iron Binding Capacity, UIBC). Due to the precise molar ratio of iron binding by transferrin, the determination of TIC can be replaced by direct quantitative measurement of transferrin.
TZhSS - reflects the content of the transferrin protein in the serum (see “Transferrin (Siderophilin)”, which transports iron in the blood. Under physiological conditions, transferrin is saturated with iron by approximately 30% of the maximum saturation possibility. The LZhS indicator reflects the amount of iron that can attach transferrin, to achieve maximum saturation. Determination of this iron is carried out after saturation of transferrin by adding excess iron (ferric chloride is added). Unbound iron is removed and bound transferrin is treated with acetic acid, after which the iron is released. This iron is reduced with hydroxylamine and thioglycolate. Next is carried out counting of reduced iron. It is possible to determine unbound iron ions by reaction with ferene. The difference between the amount of excess iron ions (unbound to iron-binding sites) and the total amount of iron ions added to the serum is equal to the amount of iron ions bound to transferrin, which is expressed as the LVSS of the blood serum .
An increase in PVSS is observed in iron deficiency anemia, in contrast to other types of hypochromic anemia. This increase in transferrin content in iron deficiency anemia is associated with an increase in its synthesis, which is a compensatory reaction in response to tissue iron deficiency.
We need to leave something for others...
At the same time, transferrin cannot take on all the iron available in the body (normally from 30 to 40% of its maximum capacity), and if the transport protein is saturated by more than 50%, then the remaining amount of Fe contained in the serum is saturated. it leaves for other proteins (albumin, for example). In this case, it is clear that, having been saturated with the element by approximately a third, transferrin still left a lot of free space (60 - 70%). These unused capabilities of the “vehicle” are called the unsaturated or latent iron-binding capacity of the serum or simply – LVSS. This laboratory indicator can be easily calculated using the formula:
- LZHSS = OZHSS – Serum Fe
LZhSS makes up ≈ 2/3 (or about 70%) of the total capacity of OZhSS. The average value of the latent iron-binding capacity of serum is ≈ 50.2 mmol/l.
Based on the results obtained when determining serum iron and the total iron-binding capacity of serum, we can find the values of CST - the coefficient of saturation of transferrin with iron (the percentage of Fe in the total iron binding capacity):
- CST = (Serum Fe: THC) x 100%
The norm of the saturation coefficient in percentage terms is from 16 to 47 (the average value of the norm is 31.5).
To help the reader quickly understand the values of some indicators that reflect the metabolism of such an important chemical element for the body, it would be advisable to place them in a table:
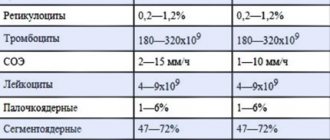
| Fe metabolism indicators | Norm |
| Serum iron (SI) | 14 - 25 µmol/l |
| OHS: men women (non-pregnant) pregnant women: I trimester II trimester III trimester | 40.6 - 62.5 µmol/l 40.8 - 76.7 µmol/l 42 - 73 µmol/l 54 - 93 µmol/l 68 - 107 µmol/l |
| LZhSS | 47 — 57% |
| CST | 16 — 47% |
It should be noted that WHO recommends slightly different (more expanded) limits of normal values, for example: CVSS - from 50 to 84 µmol/l, LVSS - from 46 to 54 µmol/l, CST - from 16 to 50%. However, the reader’s attention was already focused on these issues at the beginning of this article.
Total serum iron binding capacity (TIBC) (includes determination of iron, TIBC)
Total iron binding capacity of serum (TIBC) is a calculated parameter that reflects the amount of iron that can be transported by blood throughout the body.
Iron entering the body is absorbed mainly in the intestinal tract and temporarily accumulates in cells in the form of ferritin. Ferritin provides a soluble protein shell to store the complex of insoluble iron(III) hydroxide and iron(III) phosphate. If necessary, iron is released into the blood and distributed throughout the body using transferrin.
Typically only 25-30% of transferrin is saturated with iron. The additional amount of iron that can be bound is the latent iron binding capacity (IBC). Total iron binding capacity (TIBC) can be indirectly determined using the sum of serum iron and TIBC.
Serum iron, total iron-binding capacity, and percent transferrin saturation are widely used to diagnose iron deficiency, but these values may vary in other body conditions.
In what cases is research usually prescribed?
- for symptoms of iron deficiency;
- when monitoring patients undergoing treatment for iron deficiency;
- in case of deviations from the norm in the general blood test;
- if hemochromatosis or iron overload is suspected.
What exactly is determined during the analysis process?
OZHS is a calculated indicator for which the level of iron and transferrin in the blood is measured and the latent iron-binding capacity is calculated.
What do the test results mean?
The results of the analysis are usually evaluated together with other indicators of iron metabolism. An increase in PVSS values may indicate iron deficiency anemia, late pregnancy (due to increased transferrin and decreased iron levels), acute hepatitis.
A decrease in life-sustaining lifespan is possible in the following conditions:
- lack of protein-synthesizing function of the liver (for example, with cirrhosis)
- autoimmune and rheumatic diseases;
- hereditary hemochromatosis;
- thalassemia is a genetic disease in which the structure of hemoglobin changes;
Test deadlines.
Usually the test result can be obtained the next day after the blood is drawn.
How to prepare for the analysis?
You should adhere to the general rules of preparation for taking blood from a vein. Detailed information can be found in the corresponding section of the article.
Changes in life insurance under different circumstances
Since this work is devoted to the general iron-binding capacity of serum, we should first of all identify the conditions when the level of the described indicator is increased and when it is decreased.
So, the values of the life-sustaining lifespan are increased in cases of the following conditions (they will not necessarily be associated with any pathology):
- Hypochromic anemia;
- During pregnancy, the longer the period, the higher the indicator (see table);
- Chronic blood loss (hemorrhoids, heavy periods);
- An inflammatory process localized in the liver (hepatitis) or irreversible replacement of the liver parenchyma with connective tissue (cirrhosis);
- Erythremia (polycythemia vera - Vaquez disease);
- Lack of a chemical element (Fe) in the diet or if its absorption is impaired;
- Taking (long-term) oral contraceptives;
- Excessive intake of iron into the body;
- Ferrotherapy (iron treatment) for a long time;
- When blood transfusions cease to be rare (hematological pathology).
Also, the total iron-binding capacity of blood serum may normally have higher values in children than in adults.
Meanwhile, there are a lot of diseases when the life-sustaining lifespan tends to decline (the life-sustaining life-saving index is reduced). These include:
- Diseases that are called anemia, adding to them the definition: hemolytic, sickle cell, pernicious;
- Hemochromatosis (a multisystem hereditary pathology called bronze diabetes, which is characterized by high absorption of Fe in the gastrointestinal tract and subsequent distribution of the element throughout tissues and organs);
- Thalassemia;
- Pathology to which the word “chronic” is applied: infection, intoxication with iron preparations, liver pathology with a disorder of its functionality;
- Malignant tumor process;
- A decrease in the amount of total protein in the blood caused by a disease (cancer pathology, nephrotic syndrome) or a sharp decrease in proteins in the diet (fasting);
- Hemosiderosis (metabolic disorder, excessive accumulation of hemosiderin in body tissues, often developing after frequent blood transfusions;
- Iron deficiency conditions.
OZHSS is one of the important tests
TIBC, or total serum iron binding capacity, is a measure of the amount of iron in your body. The transport of this element is transferrin; OZhSS is one of the tests that helps determine its content. An increased concentration indicates iron deficiency, a decreased concentration indicates the opposite. These two phenomena may indicate serious illness, so consulting a doctor is necessary. A hematologist deals with blood problems, and a therapist can also give the necessary recommendations. That's all, I wish you good luck and good health!
Is increased latent iron-binding capacity dangerous?
If the latent total iron-binding capacity is increased, then specialists decide to prescribe appropriate treatment, which will depend on the results of the analysis. Basically, the need for such an analysis arises in the following cases:
- There are deviations from the norm in the blood test results. In this case, a blood test can be carried out to determine the concentration of hemoglobin, the number of red blood cells, etc.
- If there is a suspicion that there is a deficiency or excess of iron in the body.
- Suspicion of hemochromatosis. It is accompanied by various symptoms, ranging from general weakness and fatigue, to irregular heartbeat and decreased sexual desire.
Reasons for increasing the life insurance ratio
If the blood pressure level is elevated, we are not always talking about pathology. For example, during pregnancy or heavy periods, this is a variant of the physiological norm. There are two options when a pathological increase in the indicator occurs: with iron deficiency in the body, when the amount of transferrin increases in order to capture the smallest amount of iron molecules. In another case, on the contrary, the life-saving vascular resistance may be increased due to the breakdown of red blood cells, when there is too much free iron in the bloodstream.
With a simultaneous increase in TCV and transferrin, they speak of the development of hepatitis. The iron-binding capacity of serum above normal is observed in: hemochromatosis, acute leukemia, osteomyelitis, bronchopulmonary infections of any origin, lupus erythematosus, rheumatoid arthritis, lymphogranulomatosis, breast cancer, cachexia, excessive dieting or fasting, and the use of oral contraception.
What does unsaturated iron-binding capacity mean?
Unsaturated OZHS is the same latent iron-binding ability, which, depending on its level, allows specialists to diagnose the patient. So, if latent iron-binding capacity is increased, what does this mean? There are several main reasons that may contribute to an increase in this level:
- Anemia. This is the most common reason as a result of which iron in the body begins to rapidly decrease. It can be caused by poor diet or chronic bleeding. Of course, in this case the unsaturated iron-binding capacity will be increased.
- Pregnancy (third trimester). In this case, there may be a deficiency of iron in the woman’s body, since a large amount of it is spent on the development of the fetus. The life insurance premium will be increased.
- Presence of acute hepatitis.
If the total iron-binding capacity is reduced, what does this mean? In this case, experts have also identified several main reasons that can lead to a decrease in life expectancy:
- Manifested chronic diseases, such as tuberculosis.
- Chronic liver diseases, high degree burns. When the liver is unable to function normally, the amount of transferrin protein decreases significantly, which leads to a decrease in life-sustaining lifespan.
- The disease is hemochromatosis. It is characterized by a violation of the process of iron absorption, only in the opposite direction. In this case, there is an excess of iron in the body. The microelement begins to be deposited in large quantities in various organs, which in turn leads to their irritation.
- Thalassemia. A disease that is accompanied by a change in the structure of hemoglobin.
- Frequent blood transfusions or use of the wrong medications when treating iron deficiency.
- Cirrhosis of the liver.
It is worth noting that the rate of unsaturated iron-binding capacity can remain within acceptable limits, even if there is a deficiency or excess of iron in the body, but this happens in very extreme cases.
The overall iron-binding capacity is reduced - what to do? It is worth noting that this analysis is necessary in order to record a deviation from the norm, so treatment will depend on the root cause that caused the decrease or increase in iron levels.